S2.Induced dipole– Induced dipole interactions.
Vander Waals bond, London dispersion force and induced dipole-induced dipole interactions all mean the same. This type of interaction is operative in non – polar molecules and it is the weakest type of attraction.

We know that iodine exists in a solid state at room temperature. As it is solid, it is obvious that the iodine molecules must be held together by some force. Iodine is a non-polar molecule and thus it does not have any dipole in it. So the question is, what binds one iodine molecule to another?
In a non-polar species, at any given moment in time, temporary dipoles can be created owing to the position of electrons. While moving in their respective orbitals, there is a chance that, at a given instant, electrons aren’t symmetrically distributed around the nucleus. Thus, a partial negative charge(-δ) is developed at the end, where there are more electrons.If there are more electrons on the right side, the right side will develop a partial negative charge. Naturally, the other end becomes a partially positive end(+δ). This positive end will now attract the electron density of the adjacent species, towards itself. As a consequence, a dipole will be induced in the adjacent species as well. Thus, a dipole in one induces a dipole in other species. This continues and accounts for the induced dipole-induced dipole interactions. The bond thus created is called Vander Waals bond and the force that binds together the atoms/molecules is called London dispersion force (as it was developed by Fritz London in 1930).

Polarizability(α)
Polarizability is the ability of a species to form instantaneous dipoles i.e it is a measure of how easily the electron cloud can be distorted.

Larger the size of the atom, more loosely are the valence electrons held. In large molecules, the valence electrons encounter less nuclear force and are away from the nucleus. Thus, it becomes easy to distort them. Thus, the polarizability of bigger atoms/ions is more than smaller ones. The Vander Waal bonds have a more pronounced effect in larger atoms/ ions/ molecules. Note that –
size of the species gets bigger→large α →the Vander Waal bond gets stronger→ →increase in melting and boiling points of these species.
As seen in the relationship below, the energy of a Vander Waal bond is directly proportional to the square of polarizability and inversely proportional to the sixth power of the dipole separation-
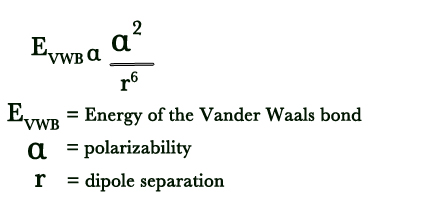
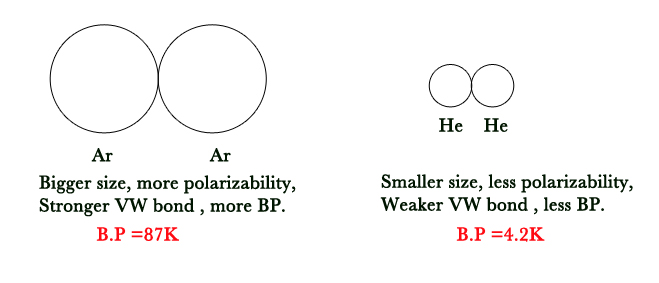
Q: Which of the two has a greater boiling point – Argon and Helium?
A: Argon is bigger than Helium.
In the next post, we start talking about the most important secondary bond. Till then, be a perpetual student of life and keep learning…
Good Day!
Image source –
1.https://www.amazon.co.uk/Iodine-crystals-resublimed-quality-product/dp/B00BXKKXAI
I really enjoyed it thanks
LikeLike
🙂
LikeLike