In this post, we start discussing a type of secondary bonding, without which life would not be possible !! How is hydrogen bonding and life on earth related?
- Water molecule has hydrogen bonds in it.Without these bonds water would not have the properties it possess. It would not be a universal solvent and would not exist as a liquid at room temperature!! 72% of our body is made of water.Can we imagine life without water ? No!!!!
- Hydrogen bonds hold the DNA strands (double helix structure) together. DNA as we know, is the genetic material present in all living things.It is thus rightly called the blueprint of life.
- Enzymes and antibodies in our body also have hydrogen bonding in their structure.
- Hydrogen bonds are responsible for linking polypeptide chains in proteins.
So then what are these hydrogen bonds?
A hydrogen bond is the partial electrostatic attraction between a hydrogen atom and an electronegative atom – nitrogen/oxygen/ fluorine. All these three atoms – nitrogen, oxygen, and fluorine have lone pair of electrons, have a smaller radius, and are much more electronegative (χ>≈3) than the H atom.
Thus, a hydrogen bond can be shown as –
A— H …. B , where,
A, B ⇒ N,O or F atom.
This implies that A and B are sufficiently close together for a secondary bond to exist between them. H atom is covalently bonded to A atom and the hydrogen bond exists between the H and B. However, the hydrogen atom has only one orbital (1s). This implies that the hydrogen bond is not an ordinary covalent bond. This is a weak bond as compared to an ionic or covalent bond. The bond energy of the hydrogen bond = 2-10Kcal/mol.
A— H ⇒ Short normal covalent bond.
H…..B⇒ Long weaker hydrogen bond.
In a typical hydrogen bond, the A— H …. B system is usually linear and unsymmetrical i.e the H atom is nearer to one nucleus than the other.
Exceptions → Salts of type M+HA2- where the bond is symmetrical. A is fluoride ion or anions of certain organic acids e.g. acetic acid or benzoic acid.
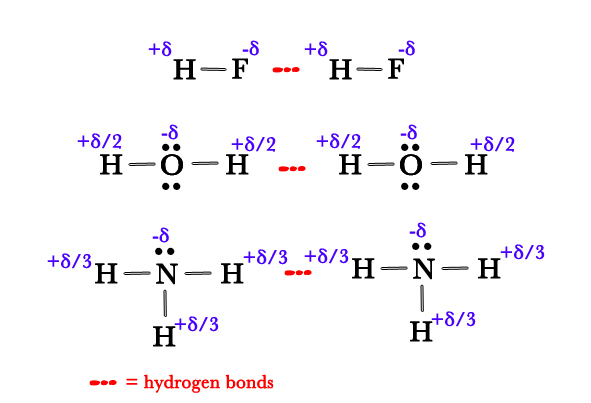
As seen in the above figure, there are partial positive charges(+δ) on H atoms and partial negative charges (-δ) on F, O, and N atoms(non-metallic elements). The partial positive charge on H atoms in a water molecule is +δ/2 as there are two H atoms and only one O atom. Thus, the net charge is neutral i.e water molecule is a neutral molecule. The same explanation holds true for ammonia too, where the partial positive charge on each H atom is +δ/3, as there are three H atoms on one nitrogen.
Why are the following bonds not considered hydrogen bonds?
B— H —B ⇒ This is not a hydrogen bond as the electronegativity of Boron(χboron=2.04) is less than H(χhydrogen=2.2).
W— H —B ⇒ This is not a hydrogen bond as Tungsten is a metal.
Since H- bonds have a low bond energy and low activation energy, they play an important role in many reactions at normal temperatures. The H- bonds determine the chemistry of water, aqueous solutions.
In the next post, we will continue our discussion on hydrogen bonds. Till then,
Be a perpetual student of life and keep learning…
Good Day!
Image Source –
Great blog I ennjoyed reading
LikeLike
Thank you!
LikeLike