I take immense pride in writing the 100th post on this blog. I am glad that I could continue writing throughout my pregnancy after my daughter was born, and today when she is 15 months young. Looking after her single-handedly and writing was a tough task! If not for my husband, I could not have thought of continuing to write. I want to thank him from the bottom of my heart, for being so supportive!
Let us now continue with our topic from the last post – hydrogen bonding. In this post, we will start to take a look at the theories enunciated to explain hydrogen bonding.
THEORY 1 – THE ELECTROSTATIC APPROACH.
In 1590, Pauling, in his book Nature of the chemical bond, has stated how H – bond is formed by electrostatic attraction.
- This approach is based on the fact that H- bonding occurs only between H and very electronegative atoms. The electronegativities of N, O and F are all more than 3 on the Pauling’s scale. Thus, these three elements form H-bonds with hydrogen atom.
- Consider a covalent bond between an electronegative atom A and H.The electron density between them will be distorted.There will be greater concentration of electron density near A than H(refer to post #46 to know why). Thus, a strong dipole will be produced.
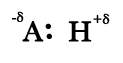
A second electronegative atom B attached to another atom or molecule will be at the positive end of this dipole.
- If the two dipoles approach one another along a straight line A— H …. B , then the electrostatic attraction between the positive end of the dipole on A—H and negative charge on B will be greater than the repulsive forces between like charges. Thus, hydrogen bond is like dipole- dipole interaction.
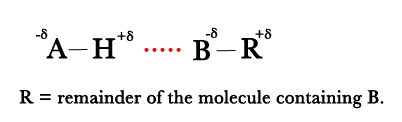
- Support for this viewpoint comes from the fact that the strongest hydrogen bonds are formed in systems in which hydrogen is bonded to the most electronegative elements.(F is the most electronegative )
F– + HF → FHF– , ΔH = – 155 KJ/mol
(CH3)2CO + HF → (CH3)2CO… HF, ΔH = – 46 KJ/mol
H2O + HOH → H2O … HOH(ice), ΔH = – 25 KJ/mol
As seen above, the strongest H-bonds are formed when A and B are fluoride ions. These have the least enthalpy (ΔH) values. H- bonds involving the O atom are the next in strength and the least strong are H- bonds with the N atom.
- The simplistic electrostatic model qualitatively accounts for relative bond energies and the geometry – A linear arrangement maximizes the attractive forces and minimizes the repulsions.
THEORY 2 – THE VALENCE BOND TREATMENT.
- This theory was developed by Coulson and Daniesson.
- It takes into account the covalent contribution to hydrogen bond by considering the following structures –
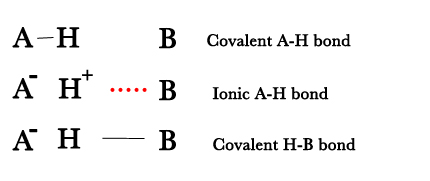
- The covalent contribution may be apprciable especially in short H-bonds.
- The reasons to believe in covalent contribution are –
i)H-bonds are short, indicating that there is considerable overlap of Vander Waas radii.This should lead to considerable repulsive forces.However, as there is no repulsion, there must be something compensating it.
ii) Symmetrical hydrogen bonds of the type F—H—F would not be expected if the hydrogen atoms were covalently bound to one F and weakly attracted by an ion-dipole force to the other. Thus, there has to be resonance i.e delocalisation of the covalent bond on both sides of the H-atom.The covalent bond can be thought to have smeared over all three atoms- A,H and B as follows –
F—H …..F– ↔ F–… H— F
THEORY 3 – THE MOLECULAR TREATMENT.
- This approach was developed by Pimentel who discussed H- bonding in
HF2– ion using MOT.
- Consider the linear combination of atomic orbitals of H (1s) with fluorine p- orbitals directed along the bond (pA and pB) . With these three atomic orbitals we get three MOs.Thus , for symmetrical F-H…F bond ,
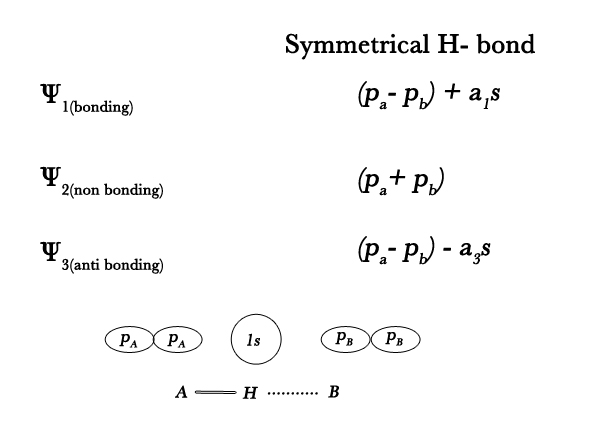
a1, a3 are mixing coefficients. The two electrons – one from H and one from F – involved in bond formation, occupy the bonding MO. This orbital spreads out axially on either side of the H- atom, thus forming two equally weak bonds. We won’t get into the details of this approach as it is beyond our course of study.
We will study the physical consequences of H- bonding in the next post. Till then,
Be a perpetual student of life and keep learning….
Happy 2019 !!😊😊😊
References and Further Reading –