We studied hydrogen bonding in the last two posts. In this post, we will study the types of these bonds and their implications. The H-bond, as we know, is formed between H -atom and the N/O/F atom. Thus, there is a strong polarity in the covalent molecules that causes this bonding.
Hydrogen bonding can be of two types –
1)Intermolecular H- bonding – Here, the H- bonding happens between two different molecules. As seen in the previous posts, two water/ammonia molecules are held together by intermolecular hydrogen bonding.

2)Intramolecular H-bonding – In some molecules, H- bonds are formed within the molecule itself. This type of H- bonding is called intramolecular H- bonding. This happens when two functional groups in the same molecule can form H- bonds with each other.
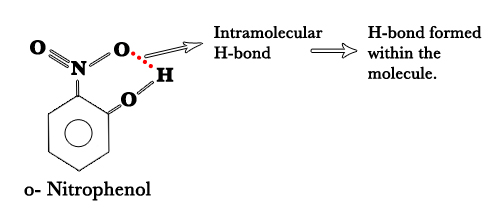
What are the physical consequences of these H-bonds?
Variation in boiling points.
The boiling point is the temperature at which the liquid boils and turns into vapor. It is thus the temperature, at which, the thermal energy(provided externally) overpowers the attractive forces, which hold the molecules together in the liquid state and convert them to vapor. The greater the attractive force, the more energy will be required to get the molecules apart, higher will be the BP.
The H- bonds are stronger than the Vander Waals bonds. Thus, more energy is required to separate molecules that are bonded by H-bonds. This results in elevation of boiling points.
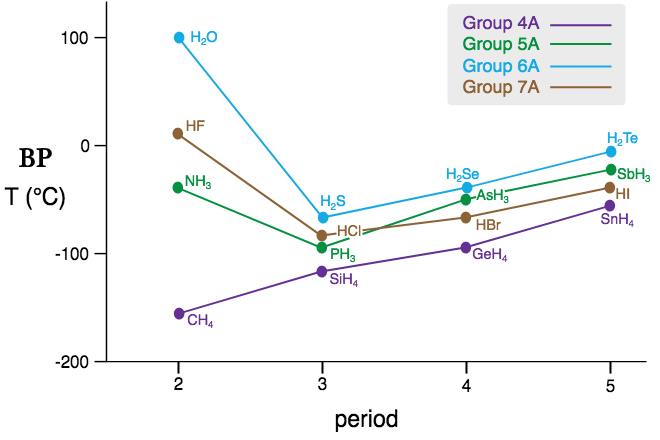
In the above graph –
• There is a steady increase in BP of Group 4A elements, as we move towards the right. The violet line shows how the boiling points increase from methane(CH4) to Stannane (SnH4). This behavior is seen on account of the London dispersion forces(LDF), which are operative here, in binding two molecules together. We know LDF increases as the size of the molecule increases. Thus,
CH4 < SiH4 < GeH4 < SnH4 …. Intensity of LDF
As the force increases, the attraction between the molecules increases too. So, it takes more thermal energy (increase in BP) to separate the molecules and create a vapor state. Thus,
CH4 < SiH4 < GeH4 < SnH4 …. BP.
• In Group 5A, 6A, and 7A, a similar trend is seen except for ammonia (NH3), water(H2O), and hydrogen fluoride(HF). As seen in the above graph, the BP of these three is more when compared to the compounds in their respective groups. This observation clearly indicates that it takes more energy to separate the molecules of these compounds as they have H-bonds in them.
• Why is BP of p-nitrophenol more than that of o-nitrophenol?
A:
BP of o-Nitrophenol = 216 º C
BP of p-Nitrophenol = 279 º C
Both o- and p- nitrophenol have H- bonds. The difference lies in the fact that o-nitrophenol has intramolecular H-bonds, whereas, p- nitrophenol has intermolecular H-bonds.


As seen above, in p-nitrophenol, there is H- bonding between two molecules, which hold them together strongly. Thus, the BP is higher than o-nitrophenol which has H- bonding only within the molecule. This, intramolecular bonding also lessens the bonding between two molecules and thus the BP is comparatively low.
In the next post, we shall continue discussing the physical consequences of H-bonding.
Be a perpetual student of life and keep learning…
Good Day!
Image source –