It gives me immense gratification to be writing this 50th post after one year of starting this blog. It gives me even more pleasure to be discussing a subject, in which I completed my Master’s degree – Organic Chemistry! So, let us continue our discussion on organic compounds in this post. We are discussing some pretty basic stuff in this post.
Before we start classifying organic compounds, it is imperative that we know the valencies of some common elements. This information is germane to our discussion as it helps us in drawing correct molecular structures.
In any organic compound –
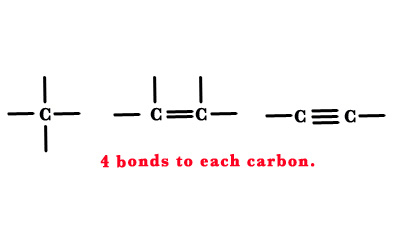
Carbon atom always forms 4 bonds. Thus, its valency is 4.
Nitrogen, Arsenic atoms form 3 bonds.
An oxygen atom forms 2 bonds.
Halogens, Hydrogen form a single bond.
|
Element | No.of bonds the element forms |
| Carbon(C) |
4 |
| Nitrogen(N), Arsenic(As) |
3 |
| Oxygen(O) |
2 |
| Halogens(-F, -Cl, -Br, -I), hydrogen(H) |
1 |

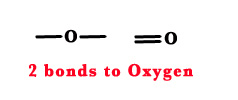

Classification of Organic Compounds
There are different ways to classify organic compounds.Some common ways are-
A] Based on structure –
The structure of organic compounds helps us classify them into different categories as shown in the figure below-
I]Open Chain/Aliphatic /Acyclic Compounds – Carbon atoms are linked to each other to form chains. Chains could be straight or branched. The word aliphatic has a Greek origin. The word ‘aleiphar‘ in greek means ‘fat/oil’. Thus, this name refers to compounds that are not soluble in water and have an open chain of carbon atoms.

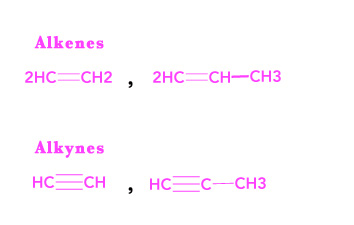
Saturated compounds have only single bonds in their structure. As shown in the figure below, they can have straight chains or can have branches. This gives rise to a wide variety of compounds. Among hydrocarbons, alkanes are saturated (have no double or triple bonds).

Unsaturated compounds have a double/triple bond in their structure.
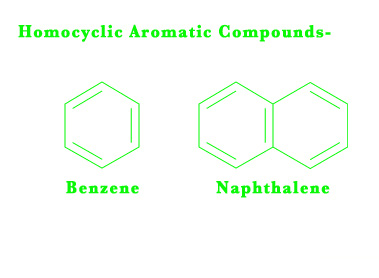
Homocyclic compounds have a closed ring structure and single bonds in them. They only have carbon and hydrogen atoms in them. They are NOT aromatic.

Homocyclic aromatic (homo= of the same kind, cyclic = ring structure) compounds have only carbon and hydrogen in their structure. They have alternate double bonds. However, these alternating double bonds impart great stability to the structure and thus aromatic compounds are very different from aliphatic unsaturated compounds. We shall study aromatic chemistry in great detail in our upcoming posts.
Alicyclic Heterocyclic compounds have single bonds in a closed ring structure of carbon atoms. However, a heteroatom (atom apart from carbon and hydrogen e.g.- Nitrogen, oxygen, sulfur, etc) is present in their structure.

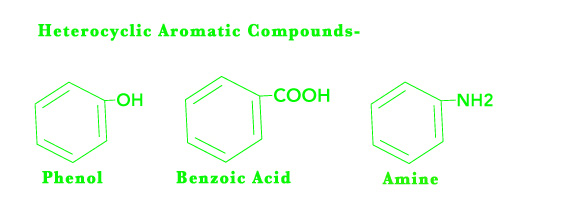
Heterocyclic aromatic compounds have an aromatic ring structure and also have a heteroatom.
B]Based on functional groups –
Organic compounds exhibit peculiar behavior based on the functional group they have. Thus, they can also be classified according to these functional groups.
What is a functional group?
It is a set of atoms that are responsible for characteristic reactions of a particular compound. The addition of a functional group changes the properties of a compound. The study of organic reactions is very intricately linked to the functional groups present in the molecule.
e.g. – Consider methane CH4. It is an alkane. If one of the -H atoms is replaced by an acidic -COOH functional group, it becomes acetic acid (CH3–COOH), which has totally different properties than methane!
So let us get to know some important functional groups in organic molecules.

Compounds containing the same functional group belong to the same class of organic compounds and their chemistry is very similar.
In our next post, we shall continue our discourse on organic chemistry. Till then,
Be a perpetual student of life and keep learning..
Good Day!
References and further reading –
1.https://en.wikipedia.org/wiki/Aliphatic_compound
2.https://en.wikipedia.org/wiki/Functional_group
3.Precise Chemistry textbook by Sheth Prakash Kendra.
Thanks for motivation.🙂👍
LikeLike
My pleasure 🙂
LikeLike