How do compounds exist in solid-state?
Compounds exist as ions in the solid-state. Consider the following example –
PCl5 molecule – This molecule has trigonal bipyramidal (TBP) geometry, which is a little less stable as compared to octahedral or tetrahedral geometry. As there are 5 substituents, this molecule is sp3d hybridized. Thus, in the solid-state, it splits into two ions as follows –

[PCl6] – ion has six substituents/ligands and so it has an octahedral geometry.
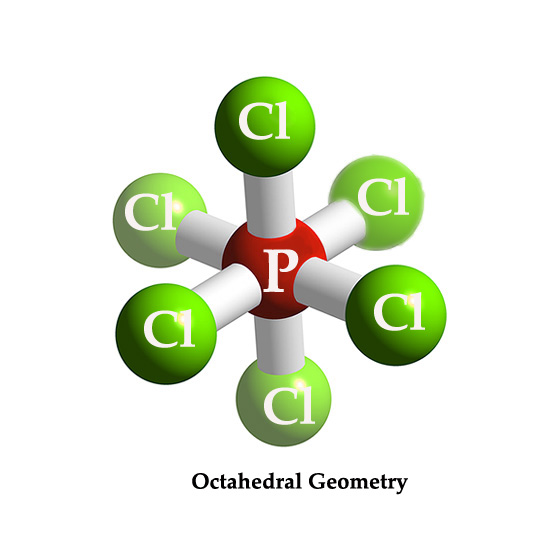
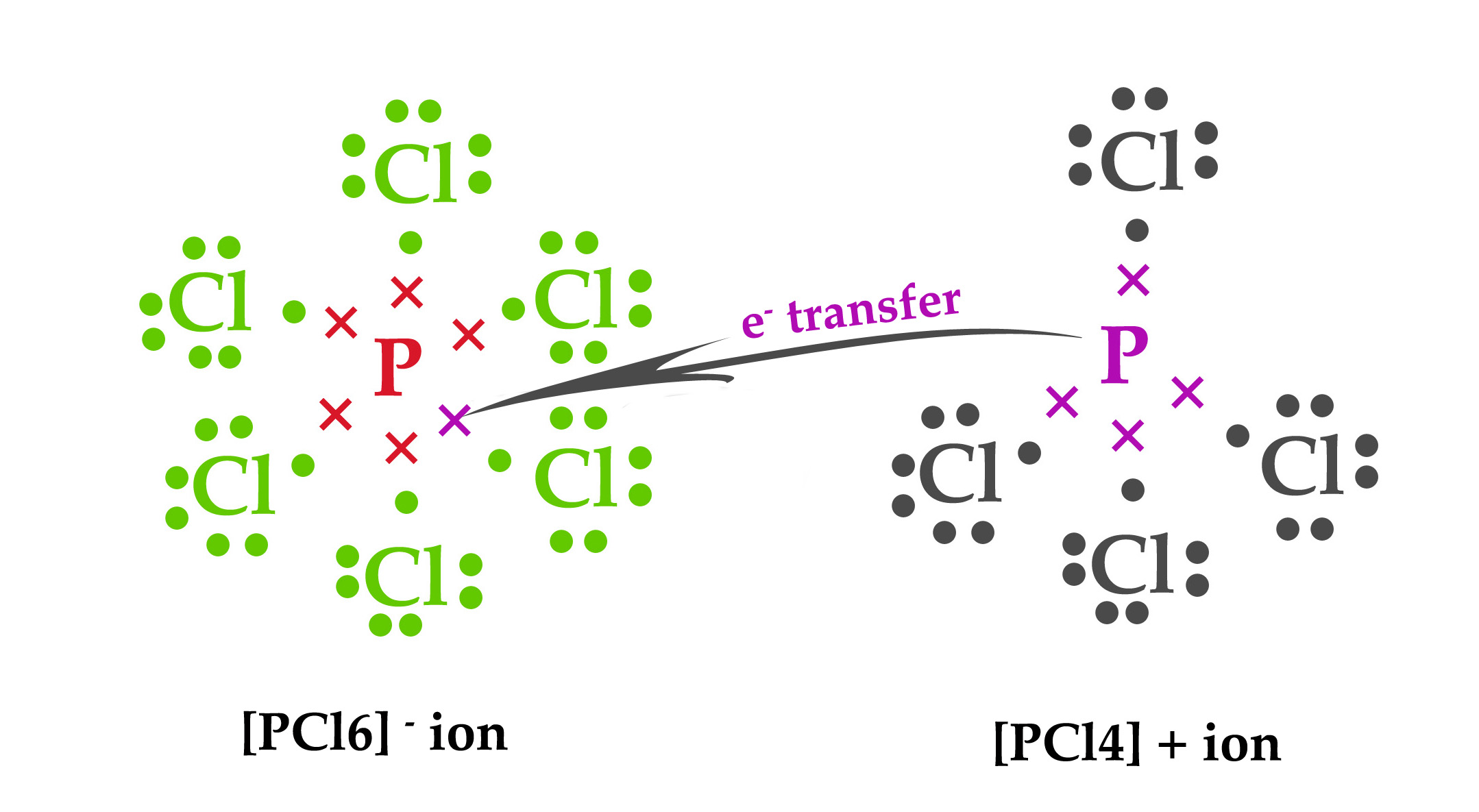
The central atom phosphorous is pentavalent i.e it has 5 valence electrons. However, in this ion, it is attached to six chlorine atoms. This is possible because a phosphorous atom can have an expanded octet i.e it has vacant valence d-orbitals, where it can accommodate an extra electron, from the sixth chlorine atom. This one extra electron, which is accommodated in the P atom’s d-orbital (which comes from another PCl5 molecule) gives this ion a negative charge.

[PCl4] + ion has four substituents/ligands and so it has a tetrahedral geometry. It is sp3 hybridized.
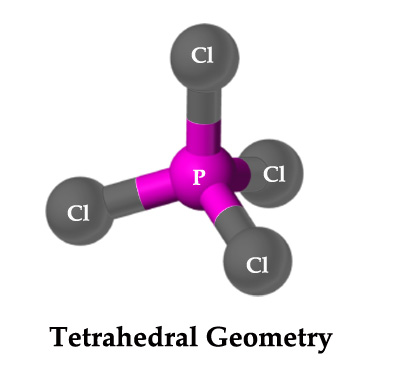
Although phosphorous is pentavalent, in this ion, it is attached to only 4 substituents. The fifth valence electron is transferred to another PCl5 molecule to form a [PCl6] – ion. As one electron is transferred to another species, the net charge on this ion is +1.

The transfer can be shown as follows –
Many compounds show this phenomenon. Some examples are –
- PBr5 →PBr4++ Br –…. Note that this molecule forms ions differently than PCl5 as the size of the bromine atom is bigger than that of chlorine. The six bigger Br atoms do not sterically fit around the P atom. Thus, we see some distortion and trigonal bipyramidal geometry. The two bromine atoms are at the axial positions, and the other three are at the equatorial positions.PI5 does not exist as iodine atoms are too large to fit into an octahedral or square pyramidal geometry.
- Cl206 → Cl202+ + Cl204–
In our next post, we shall study another rule which is important in the hybridization series. Till then,
Be a perpetual student of life and keep learning…
Good day!
References and Further Reading –
PI5 doesn’t show solid state hybridisation
LikeLike
Yes! You are right! Thank you for pointing out the mistake!! I corrected it 🙂
LikeLike