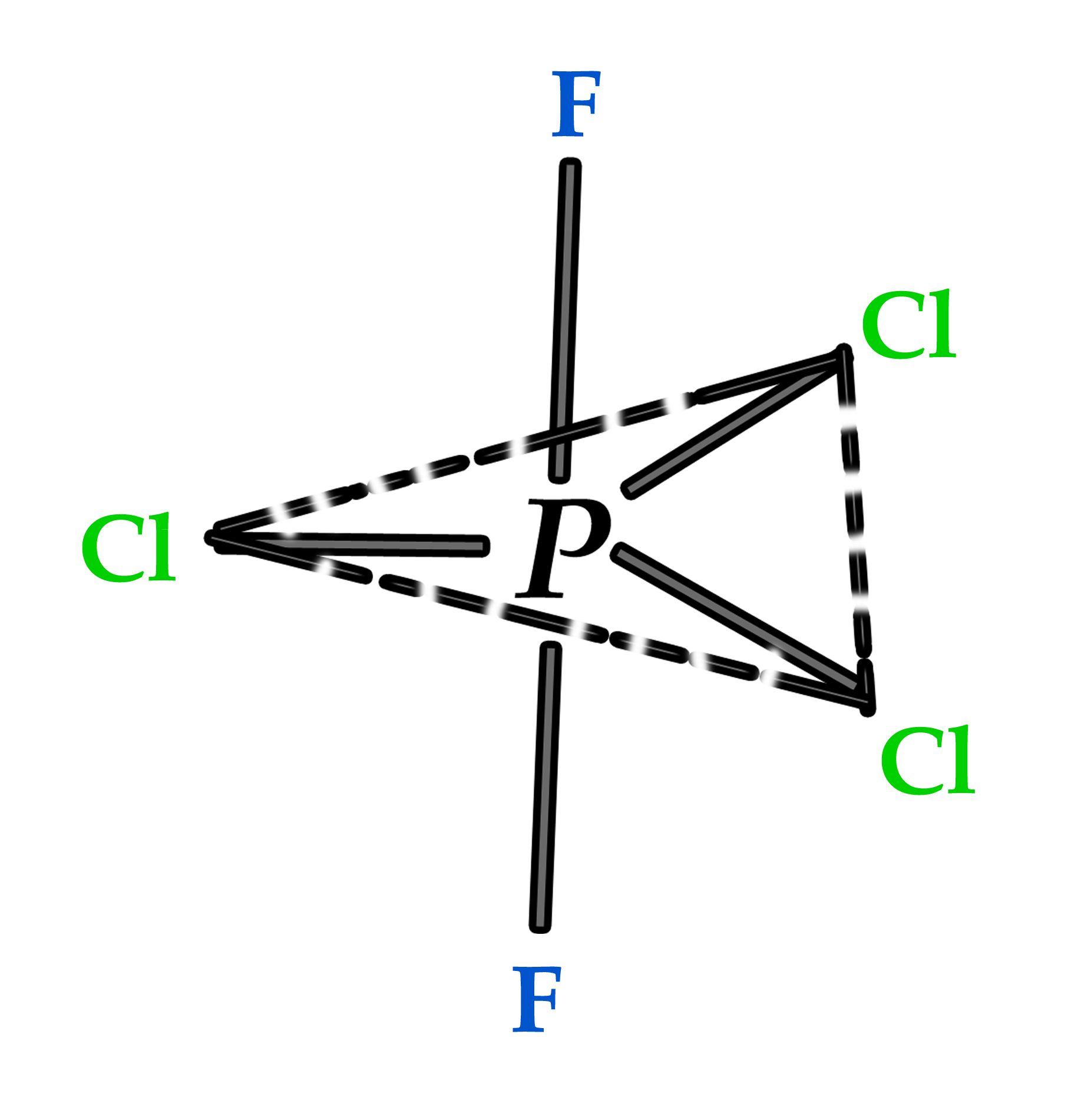
Example 3 – PCl3F2 molecule .
This molecule has TBP geometry, with two F atoms and three Cl atoms as substituents. According to Bent’s rule, the F atoms are more electronegative and so they will prefer the axial position, where there is no s-character (less electronegative). The three Cl atoms will occupy the equatorial positions. Thus, the correct structure of this molecule is as shown below –
Relationship between bond length and bond angle.

|
Name |
Average C-F bond length |
|
Fluromethane |
1.388 Å |
|
Difluoromethane |
1.358 Å |
|
Trifluoromethane |
1.329 Å |
|
Tetrafluoromethane |
1.323 Å |
Bent Rule Energetics.
Any system in the universe always tries to lower its energy as lower energy corresponds to more stability. How does Bent’s rule explain the lowering of energy?
According to Bent’s rule, in isovalent hybridization (hybridization in asymmetric molecules),
%s character is directed more towards electropositive elements &
%s character is directed less towards electronegative elements.
An electronegative substituent draws electron density away from the central atom. This results in the central atom being less stable. So, automatically, the molecule adjusts the s- character in such a way, as to render stability to the central atom. So, what happens? The s- character (which has more electron density) is directed towards the less electronegative atoms. In the bond between the less electronegative atoms(e.g.- H atoms) and carbon, carbon is more electronegative. So, now the electron density in C-less electronegative bonds gets concentrated near carbon. What happens in the overall molecule?
1)The electronegative element wants more electron density (as it has a tendency to accept electrons). So, the electrons from the C-electronegative substituent bond get closer to the electronegative element(e.g. – F). So, the electronegative element is happy! However, the energy of the bonding electrons slightly increases in this case. Let us assume that,
the increase in energy of bonding electrons = Eincrease .
2)The transfer of s character towards electropositive elements,leads to maximum bonding between C- electropositive substituent bonds. Thus, there is a decrease in the energy of the electrons in these orbitals. Let us assume that,
the decrease in energy of bonding electrons = Edecrease .
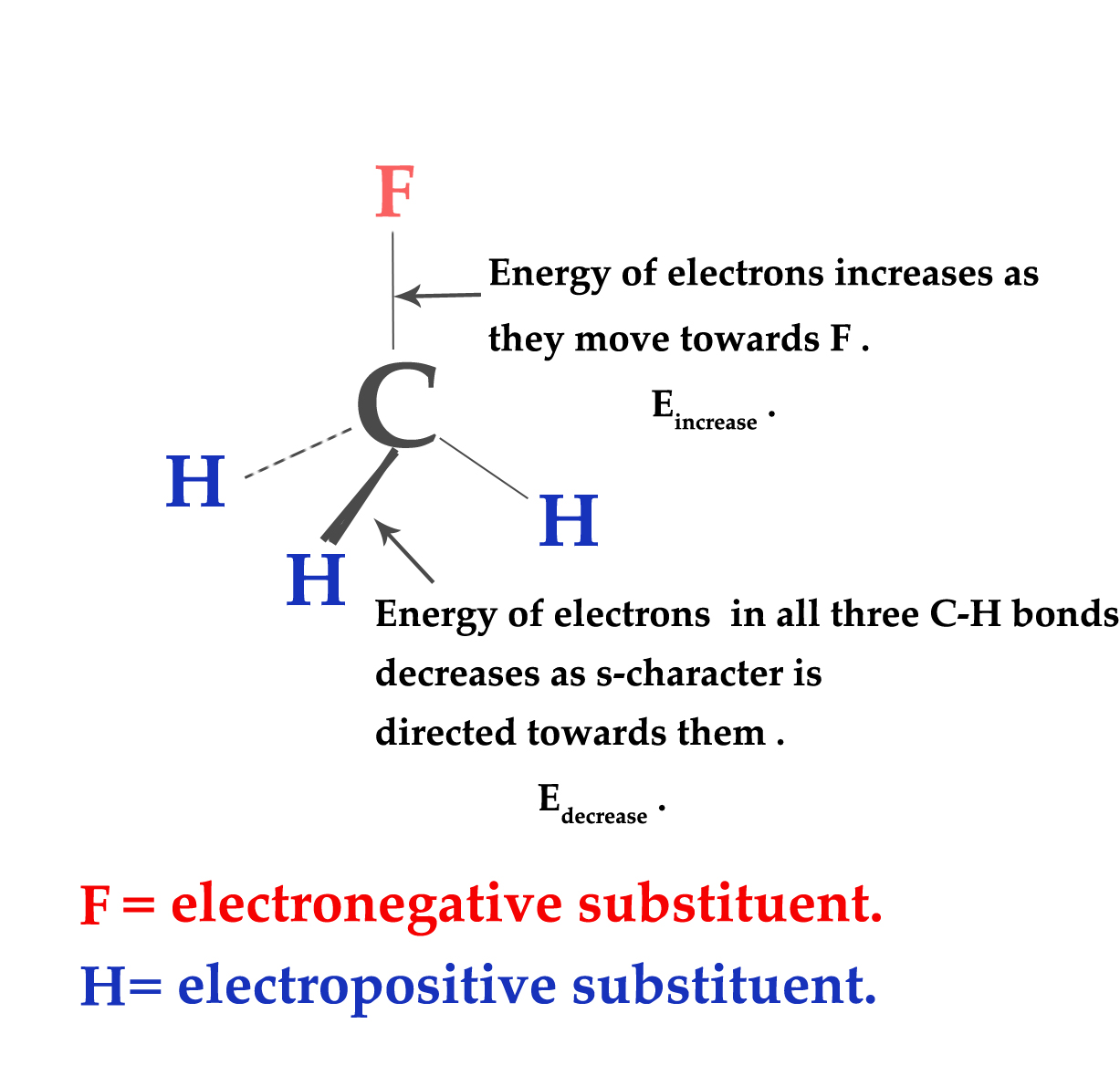
The decrease in the energy due to shifting the s-character towards the electropositive substituents is more than the increase in energy due to the shifting of electrons( in C- electronegative element) towards the more electronegative element i.e
Edecrease > Eincrease .
Eincrease – Edecrease = – ΔE
– ΔE = Net decrease in the overall energy of the system.
Thus, the overall energy of the molecule becomes less( the negative sign on ΔE indicates there is a decrease in energy ) and the molecule tends to be more stable.
Loved the content. So much easier to comprehend this. Thank you
LikeLike
Hey Sharala.. I am glad I could be of help:)
LikeLike