Overlap & Symmetry
Bonds have different symmetries, based on how they are formed. Bonds in molecules can have the following symmetry –
i) Sigma (σ)
ii) Pi (π )
iii)Delta (δ) or
iv) Phi (Φ)
Sigma Symmetry
A sigma bond is formed when two s or two pz orbitals overlap. Both these orbitals are symmetric around the internuclear axis. The bond formed is symmetric around that axis too. Thus, rotation around the internuclear axis brings about no change in the phase of the orbital i.e the sign of the orbital does not change.
A sigma orbital is formed by s or pz orbitals.
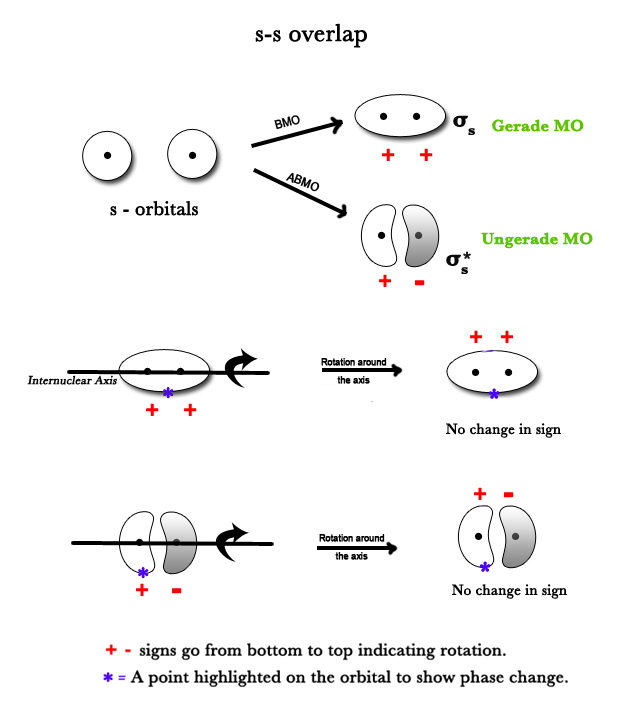
Two s- orbitals overlap to give a BMO and an ABMO.Both have sigma symmetry as they are symmetric around the internuclear axis. (The ‘∗‘ sign is drawn just to highlight a point in the orbital. The point is in the positive lobe before and after rotation indicating there is no phase change).
Similarly, we can show sigma orbitals for pz orbital too.

NOTE – Gerade and ungerade terms have to do with an inversion around the inversion center and sigma symmetry has to do with symmetry around the internuclear axis.
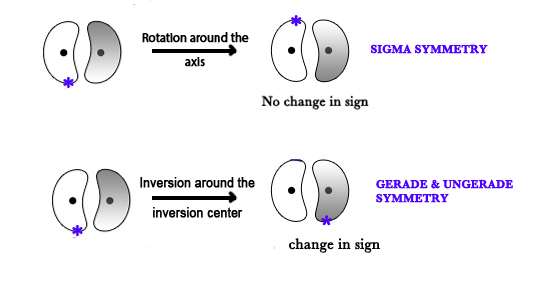
Only pz orbitals are involved in σ bond formation. The px and py orbitals form π bonds.
In the next post, we will continue studying this topic further. Till then,
Be a perpetual student of life and keep learning…
Good day!
References and Further Reading –