Pi (π) Symmetry
Orbitals with π symmetry are NOT symmetric w.r.t to the internuclear axis. When rotated around the axis, they produce a phase change (which means +sign changes to – and vice versa). The px and py orbitals form π bonds, perpendicular to the internuclear axis.
This is because the px and py orbitals lie along the x- and y-axis, which is at 90º to the plane containing the internuclear axis. The pz is in the plane of the internuclear axis as shown below. This is just a convention created for avoiding any confusion.
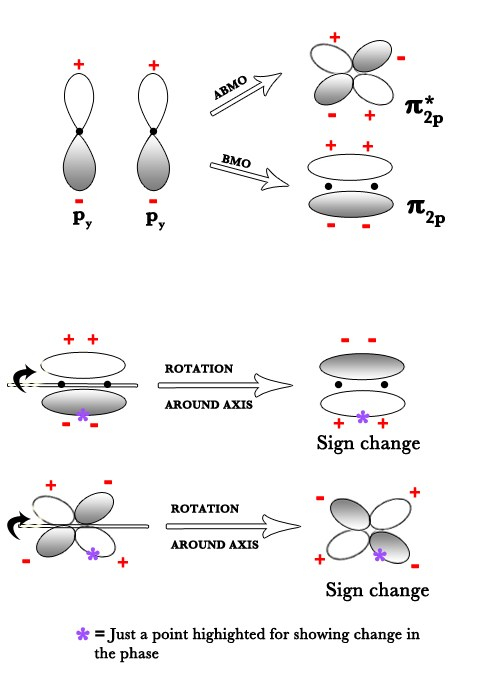
Two py orbitals overlap to form one BMO and one ABMO. Both orbitals are not symmetric w.r.t the internuclear axis as when rotated around it they produce a phase change as seen in the figures below. However, the BMO has gerade symmetry(πg) while the ABMO(π*u) is ungerade. This holds true for the px orbital too.
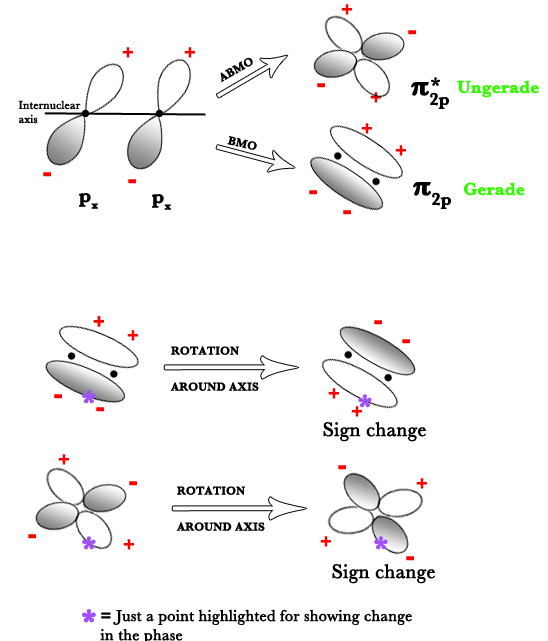
A similar diagram can be shown for px orbital too.
The π bonds are formed by the lateral overlap of orbitals and these are weaker than the sigma bonds. This weakness can be attributed to the fact that the pi bonds do not fall in the region of the internuclear axis where the electrostatic force of attraction from the nucleus is maximum. The overlap is less as compared to sigma overlap too and so pi bonds are easy to break. Thus, mostly compounds with π bonds (double and triple bonded atoms) are reactive (e.g.– alkenes, aldehydes, ketones, alkynes, etc).
Delta Symmetry
A MO with δ symmetry results from the interaction of two atomic dxy or dx2-y2 orbitals. These MOs are generally found in transition metal complexes. We shall not discuss these in detail as the structures of d- orbitals are slightly more complex.
Phi Symmetry
This is a rare type of symmetry found in f- orbitals. As of now, only one molecule is known to contain this symmetry – a U−U bond, in the molecule U2).
We almost always majorly encounter interactions between s- and p- orbitals only and so a clear view of sigma and pi symmetries is necessary.
In the next few posts, we will draw MO diagrams for various molecules to understand the MOT clearly. Till then,
Be a perpetual student of life and keep learning...
Good Day!
References and Further Reading –