Helium molecule (He2) –
He (2) – 1s2.
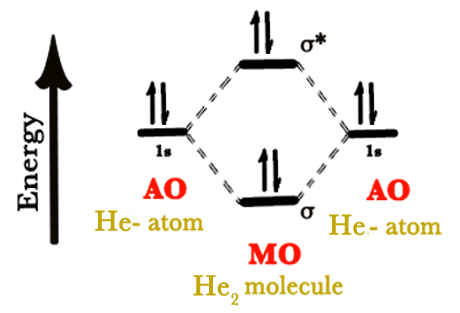
Helium has two electrons in the 1s orbital. Thus, when we draw the MO diagram, two electrons occupy the BMO and the other two have to occupy the ABMO.

∴Bond order = 1/2 (2-2) = 0.
The bond order is zero which indicates that there is NO bond between two helium atoms. A He2 molecule does NOT exist. The bond between two helium atoms is so weak, that it breaks even if the molecule vibrates or rotates. The thermal energy at room temperature is sufficient to rotate and vibrate the bonds in this molecule.
HELIUM MOLECULE DOES NOT EXIST.
Before proceeding further we need to have a look at the following figure –

The above figure shows the s and p orbital mixing in the second-row elements of the periodic table. These second-row elements have s and p orbitals as their valence orbitals, which take part in bonding.
As seen in the above figure, the diatomic molecules like Li2 , Be2 , B2, C2 and N2 the energy gap between the 2s and 2p is less. This is because the effective nuclear charge(Zeff) increases as we move from left to right in the second row of the periodic table.
What is the effective nuclear charge, Zeff ?
The outer/ valence electrons of an atom with more than two orbitals do not experience the full nuclear pull from the nucleus of that atom as the inner electrons shield/protect the outer electrons from the nuclear charge. In other words, the inner electrons don’t let the outer electrons feel the entire pull from the nucleus. This phenomenon is called shielding.
Shielding can also be described as the net effect of repulsion between inner and outer electrons and the attraction of the outer electron by the nucleus.
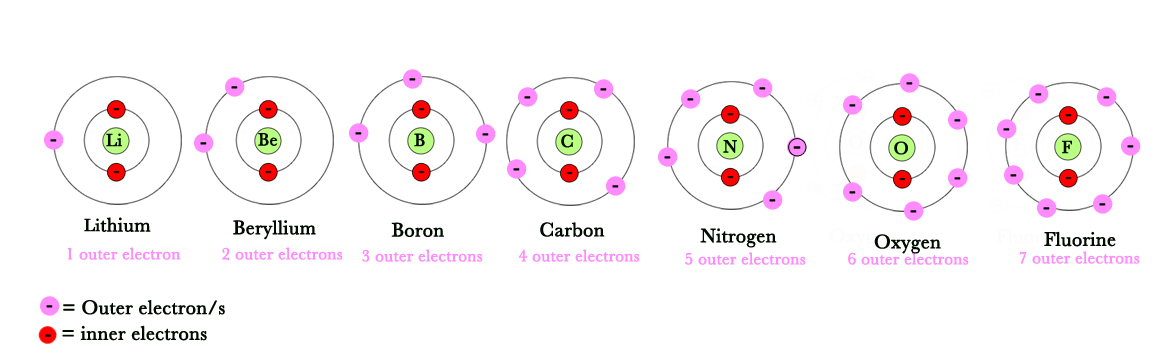
In the following figure, the two inner electrons experience the nuclear charge completely as there is nothing coming in the way between them and the nucleus. Thus, they are tightly bound to the nucleus.
However, the outer electron is shielded by the inner two electrons. Thus, the nuclear charge it experiences is far less. This net nuclear charge, that the outer electron experiences, is called the effective nuclear charge, Zeff .


What happens as we move to the right along the second row of the periodic table?
The atomic number(Z) increases, but the no. of inner electrons which are responsible for shielding (the inner s- orbital electrons) DO NOT increase. As seen below, there are only two inner electrons for second row elements. However,the # of outer electrons increases as Z increases from left to right.
inner electrons – remain constant
outer electrons – increase from left to right.
Thus, Z goes on increasing and S does not . The formula,
Zeff = Z-S
shows us that, the Zeff will increase.This is exactly what is observed experimentally.
Zeff increases across a period (due to increasing nuclear charge with no accompanying increase in shielding effect).
When the effective nuclear charge (Zeff) , is small, it means that there isn’t a great energy difference between 2s and 2p orbitals.So, 2s and 2p orbitals are closer in energy to each other and thus, the π2p orbital is lower in energy than the σ2p orbital.
From O onwards, Zeff is more. So, there is a considerable energy difference between the 2s an 2p orbital energies.As seen in the figure below, the order changes from N(Z=7) to O(Z=8) and from O onwards the σ2p orbital is lower in energy than the π2p orbitals.
In the figure below, there are two degenerate π2p orbitals (4 electrons) and one σ2p orbital, inside the red box.The positions of these two orbitals change going from Nitrogen to oxygen.
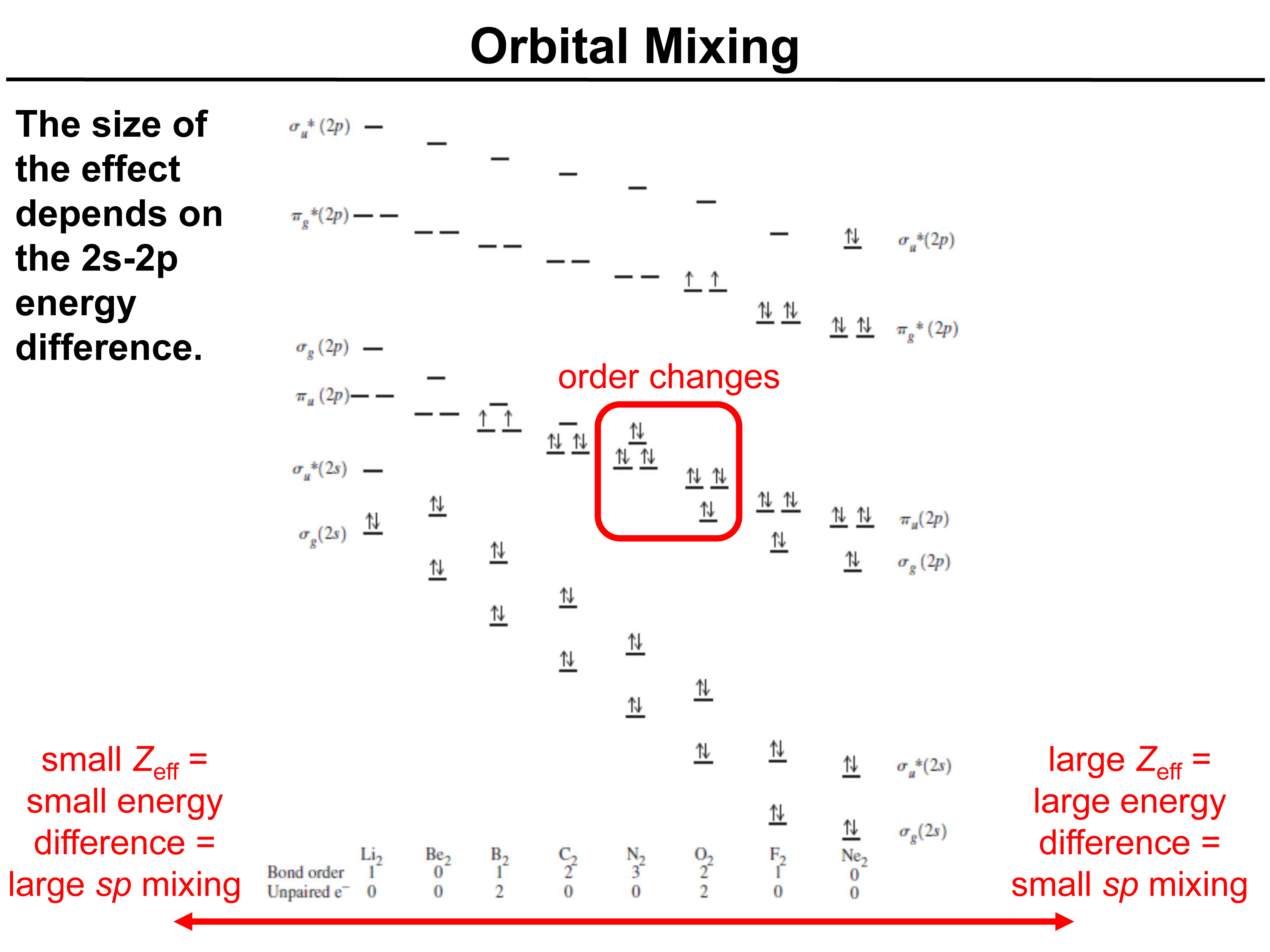
Thus, the value of Zeff helps us to determine how the atomic orbitals will mix to give us the new molecular orbitals.This is referred to as ORBITAL MIXING.
How do we remember this?
We will study nitrogen and oxygen MO diagrams in the next post and see how the diagrams differ.Till then,
Be a perpetual student of life and keep learning…
Good Day!
Image source –
- https://www.chem.uci.edu/~lawm/10-9.pdf
- https://www.google.co.in/search?biw=1280&bih=649&tbm=isch&sa=1&ei=DJMQXJGuHdqv9QPg74yYDQ&q=pie++clipart+free&oq=pie++clipart+free&gs_l=img.3..0j0i5i30l4j0i8i30l2.267208.268067..268957…0.0..0.112.505.1j4……1….1..gws-wiz-img.mRmM3MksEEg#imgrc=o4N5m8oSXURMTM
References and Further Reading –