Nitrogen molecule (N3) –
N (7) 1s2 2s2 2p3
When we construct the MO diagram for nitrogen, we only draw the valence 2s and 2p orbitals.
In the nitrogen’s MO diagram, there is a slight change when compared to the MO diagrams of O2, F2 molecules. Here, the σ2p orbital is higher in energy than the π2p orbital. This is because the σ2p orbital in oxygen/fluorine is more strongly attracted to the nucleus (owing to the higher nuclear charge). Thus, it’s more stable and has less energy. In nitrogen, however, the attraction is comparatively less and so the π2p orbital has lesser energy than the σ2p.

Electronic configuration – (σ1s)2 (σ2s)2 (σ*2s)2 (π2p)4 (σ2p)2
Each N atom has 5 valence electrons – two in 2s and three in 2p orbital. Two N atoms come together to form a nitrogen molecule and the 10 electrons fill the MOs as shown in the figure above.

∴ BO for nitrogen = 1/2 (8-2) = 6/2 = 3.
Thus, there is a triple bond between two N atoms. The triple bond makes this molecule very stable. Its bond dissociation energy is very high – 945kJ/mol.
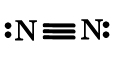
Oxygen (O2) –
The Lewis structure for oxygen can be shown as follows –
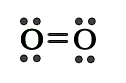
There is one sigma (σ) and one pi (π) bond between two oxygen atoms. The molecular diagram can be drawn as follows –
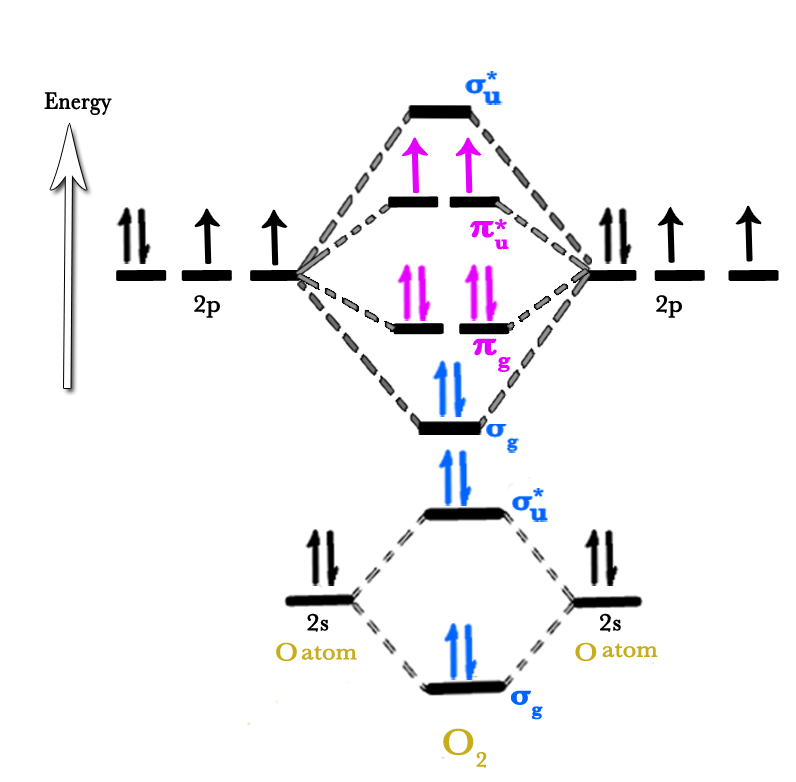
In the above figure, the electrons in sigma(σ) orbitals are shown in blue color, and the pi (π) orbital electrons in pink.
Oxygen (8) ⇒ 1s2 2s2 2p4.
- The above figure shows only the valence electrons(2s and 2p) as only they are responsible for bonding.
- Two sigma(σ) orbitals are formed. Both are shown as σg in the above figure – one of them is σ2s and one is σ2p.
- The 2s electrons occupy both bonding (σ2s) and anti bonding(σ*2s) orbitals. The electrons have to occupy anti bonding σ*2s orbital(ABMO) as the next bonding orbital(σ2p) is higher in energy than this ABMO.The σ2s bonding orbital is gerade so it is shown as σg in the figure.The σ*2s ABMO has ungerade symmetry so it is shown as σ*g in the figure above.
- The 2p AOs overlap to form bonding σ and anti bonding σ* as well as bonding π and anti bonding π* orbitals.
- Note that the energy difference between two sigma orbitals (ΔEσ) is much more than energy difference between two pi orbital (ΔEσ). This is because there is greater overlap in σ orbitals as compared to π orbitals.

 ∴Bond order = 1/2 (8 -4) = 2 .
∴Bond order = 1/2 (8 -4) = 2 .
Thus, there are two bonds between the two oxygen atoms.
Any molecule with one or more unpaired electrons is paramagnetic. As seen in the MO diagram above, oxygen has two unpaired electrons and so it is paramagnetic i.e it is attracted by external magnetic fields.
We continue studying more examples in the next post. Till then,
Be a perpetual student of life and keep learning..
Good day!
Fluorine molecule () –
F (9) 1s2 2s2 2p5
Two fluorine atoms bring 7 electrons each to the table and thus the 10 electrons get arranged in the MOs formed as follows –

Electronic configuration –(σ1s)2 (σ2s)2 (σ2s)2 (σ2p)2 (π2p)4 (π2p)4
There are no unpaired electrons in the molecular orbitals of fluorine and so it’s diamagnetic i.e it has no permanent magnetic dipole moment.

∴Bond order for F2 molecule = 1/2 ( 8-6)= 1
Thus, there is a single bond between two F atoms.
We continue studying some more examples in the next posts. Till then,
Be a perpetual student of life and keep learning…
Good Day!