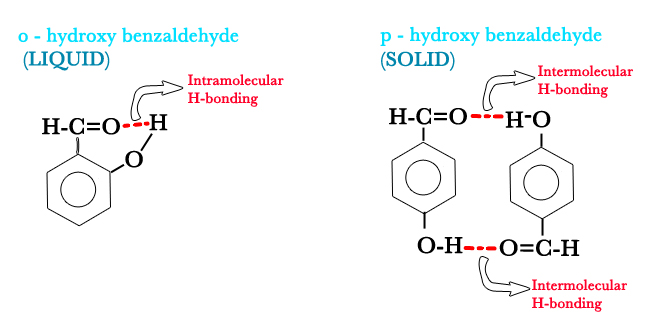
Q: Why is o- hydroxy benzaldehyde a liquid whereas p-hydroxy benzaldehyde a solid, with high MP, at room temperature?
A: o- hydroxy benzaldehyde has intramolecular H- bonds and so the molecules are not very tightly bound to each other as in the case of p-hydroxy benzaldehyde, where, the intermolecular H-bonds hold molecules together, making it a high melting solid.

Q: What type of C-C bond is highlighted in red in the figure below?
i.σ(C2sp3,C2sp3) and π (C2sp3,C2sp3)
ii.σ(C2sp3,C2sp3) and σ (C2sp3,C2sp3)
iii.σ(C2sp2,C2sp2) and π (C2sp2,C2sp2)
iv.σ(C2sp2,C2sp2) and π (Cpy,Cpy)
A: First thing to understand is that – ONE SHOULD NOT GET SCARED BY SEEING SUCH A COMPLEX STRUCTURE. The question is only related to a double bond in the structure. So let’s focus only on that part.
We know that when there is a double bond, one bond is a sigma bond(σ) and the other is a pi(π) bond. So, we can rule out option no ii as it has no π bond component. We also know that a π bond is made from p-orbitals. Thus, we can rule out options no i and iii, as they don’t have a π bond with p-orbitals. Thus, the answer to the above question is – an option no iv.
Q: Why ClF2– is linear but ClF2+ is a bent molecular ion?
A: The hybridization of these two ions are responsible for their respective structures. How do we find the hybridization? We know that,
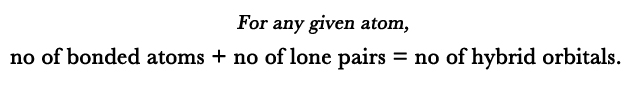
Chlorine and fluorine both have 7 electrons in their valence shell.
ClF2– ion –
∴Total no of valence electrons for ClF2– = 7 + 2(7) + 1 = 22. (We add 1 for the negative charge).
Out of the 22, 4 electrons are bonding and 18 electrons make 9 lone pairs. Now let us draw the Lewis structure of this molecule. Chlorine is less electronegative, so it will be the central atom. After two bonds are formed, we have to arrange 9 lone pairs over the three atoms. Each atom has three lone pairs as shown below –

The chlorine has 10 electrons on it, in this structure – thus Cl has an expanded octet here.
no. of hybrid orbitals for chlorine = 2 + 3 = 5. Thus, it is sp3d hybridized. So, this should have a trigonal bipyramidal geometry. But as we studied in post 73-

So, the LPs will occupy the equatorial positions. Thus, the structure will be as follows –

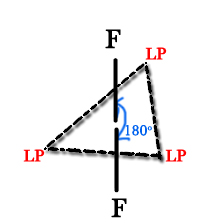
Thus, if we ignore the LPs then the molecule will look linear(180º).
ClF2+–
Total no of valence electrons for ClF2+ = 7 + 2(7) – 1 = 20. (We subtract 1 for the positive charge).
Out of 20 electrons i.e 10 pairs, 2 are bonding. Thus, we have to arrange 8 pairs on three atoms. The Lewis structure can be drawn as follows –
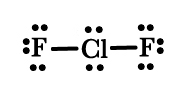
The structure in this case will be as follows –

This ion has a bent geometry as seen in the above figure.
In the next post, we shall start discussing a new topic. Till then,
Be a perpetual student of life and keep learning ….
Good day !
Nice
LikeLike