From this post onwards, we shall start discussing a phenomenon, which manifests itself in many compounds and which is responsible for deviations in structure, properties, and reaction mechanisms of organic and inorganic species.
Many a time, it is not possible to define the structure of a molecule using just one Lewis structure. There are many contributing structures that together can completely explain the structure of that species. Resonance is the description of these structures and their connection to each other. Thus,
Resonance is the description of the electronic structure of a molecule by means of several schemes of pairing electrons, with features of each scheme contributing to the description.
In simple words, this means that a species(molecule/ion) can have many resonating structures, each contributing to the overall structure of the molecule. Thus, in species where a single Lewis structure is inadequate to describe the species totally, there are many resonating structures, which together make that species.
e.g. What is the structure of Ozone (O3)?
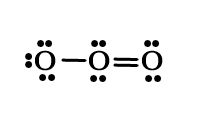
The above structure suggests that ozone has one single and one double bond. However, when the bond lengths are calculated experimentally, the value for both bonds is the same and the bond length value lies intermediate between a single and double bond!
Single bond length for O – O → 1.48Aº
Double bond length for O=O → 1.21 Aº
The bond length in ozone → 1.28 Aº
This observation suggests that both bonds are similar and show the character between single and double bonds. This can be shown by drawing two resonating structures for ozone as follows –

As seen above,
resonating structure 1 → a single bond between the first and second oxygen and a double bond between the second and third oxygen atoms.
resonating structure 2 → a double bond between the first and second oxygen and a single bond between the second and third oxygen atoms.
Both these structures contribute to the overall structure of ozone, which can be shown as a hybrid structure, as follows –
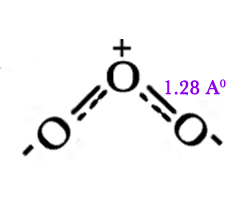
- Resonance should be pictured as a blending of structures, and not as flickering alterations between them. In quantum mechanical terms, for a molecule with n resonating structures,
Ψ(molecule) = Ψ(resonating structure 1) + Ψ(resonating structure 2) + …….. + Ψ(resonating structure n).
- Resonance has two main effects-
- 1)It averages the bond characteristics over a molecule.
2)Energy of the resonance hybrid structure is lower than any of the single contributing structures.
FORMAL CHARGE
Before proceeding to draw resonating structures for molecules, we must know how to calculate formal charges(FC) on atoms in a molecule. Ions either have a positive or negative charge. Neutral species carry zero charges. Knowing which atoms in a polyatomic species carry what charge can help us understand the charge distribution in that species. We know that the valence electrons do not belong to only one atom specifically and these electrons are not always shared equally. The distribution of electrons depends on the electronegativity of atoms in the species. A more electronegative element will pull the electrons more toward itself than a less electronegative element. Calculating formal charges help us to exactly know-
i)which atom carries what charge
ii)how much charge.
The formula for calculating FC is –
FC = VE – LPE – (BE/2)
where,
FC ⇒ Formal Charge.
VE ⇒ Valence Electrons.
LPE ⇒ Lone Pair Electrons.
BE ⇒ Bonding Electrons.
Let us understand this concept with some examples.
- Methane (CH4) – First draw the dot structure of the methane. How to draw dot structures? Read post # 53.
 Thus, the formal charge on carbon in methane is zero i.e the molecule is neutral.
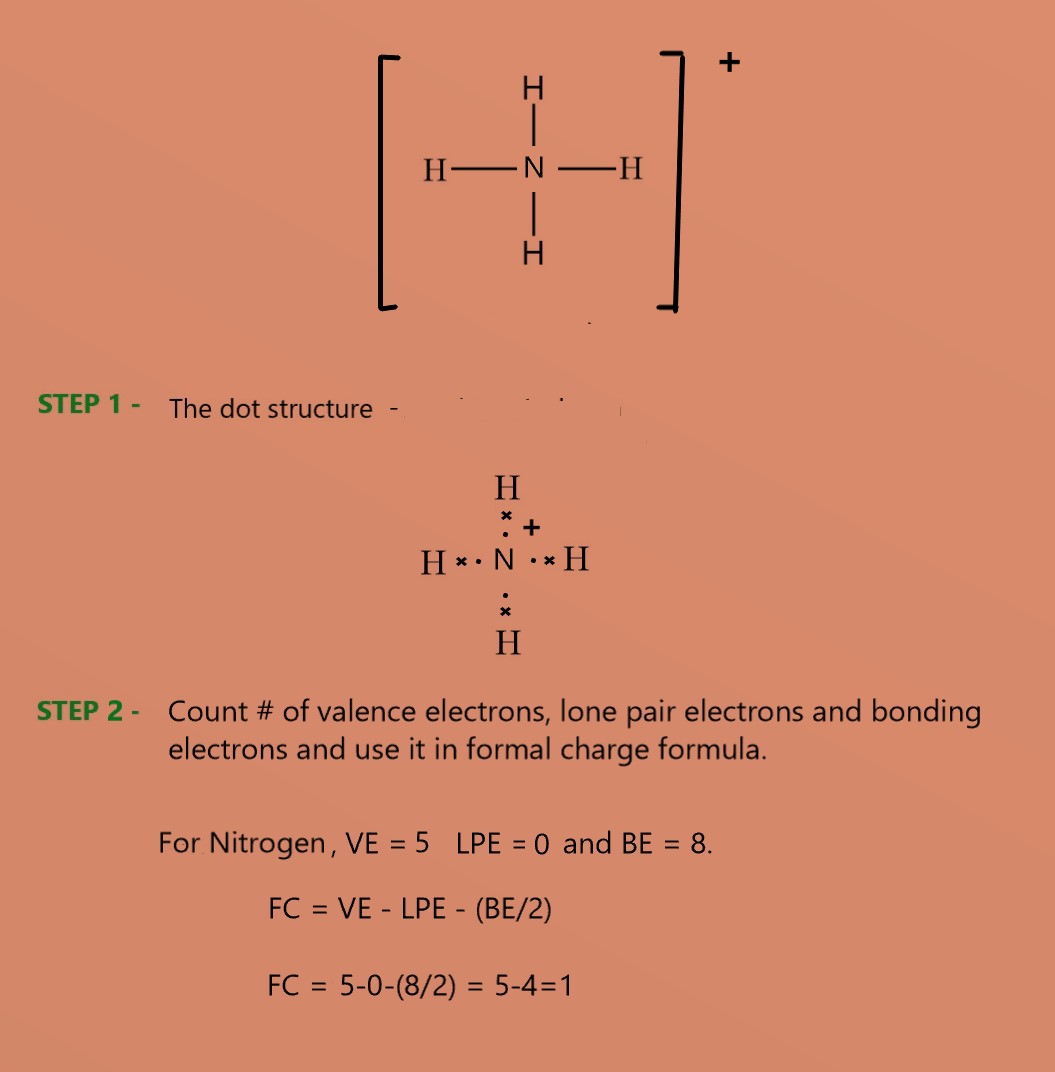
Thus, the formal charge on carbon in methane is zero i.e the molecule is neutral. - Ammonium ion (NH4+) – In this ion, there are 5 electrons on the N atom and no lone pairs. So the Formal charge on the central N atom can be calculated as follows –
Though calculating the formal charges mathematically is a foolproof method, as chemists, we can also do that instinctively. In fact, doing it instinctively is much easier! We should know the common bonding patterns of some basic elements –
CARBON – forms 4 bonds (tetravalent), no lone pair of electrons.
NITROGEN– 3 bonds (trivalent), 1 lone pair
OXYGEN – 2 bonds(bivalent), 2 lone pairs
HALOGENS– 1 bond(monovalent), 3 lone pairs
The following table shows a neutral atom and atoms with a positive and negative formal charge-
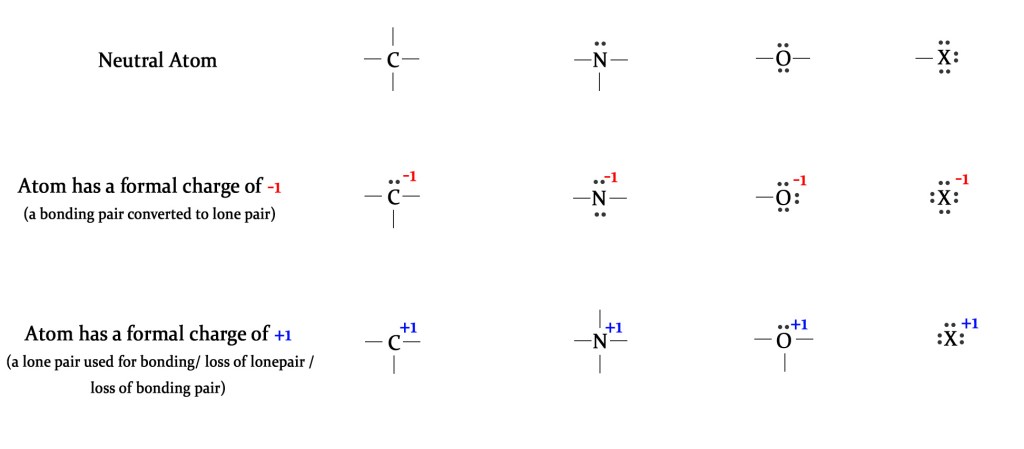
As seen above, when a bonding pair of electrons is converted to a lone pair (by accepting an electron from another atom), the central atom acquires a negative charge. When a lone pair of electrons is used for bonding with other atom/s, the central atom gains a positive charge. A positive charge is also acquired by losing a lone or bonding pair of electrons.
e.g.-
- Ammonium ion (NH4)+–

Nitrogen has no formal charge in an ammonia molecule. However, when it donates its lone pair of electrons to a hydrogen ion, it forms an ammonium ion. The formal charge on nitrogen now becomes +1( it is written outside square brackets), as shown above.
2. Carbanion ion(CH3)––

A carbanion ion is formed by the heterolytic cleavage of a carbon-hydrogen bond. In this type of cleavage, the carbon atom retains the bonding pair of electrons, and the hydrogen leaves with a positive charge on it. The carbon atom with three hydrogens and a lone pair is referred to as a carbanion ion.
We will continue our discussion on resonance in the next post too. Till then,
Be a perpetual student of life and keep learning…
Good day!