In this post, we shall study how to draw structural formulae for different organic molecules. Understanding the structural representation of molecules is very important, as it allows us to get information about the arrangement of atoms in space. This arrangement dictates the properties of molecules. Thus, these structural formulae are a roadmap to fully understanding molecules.
To emphasize the importance of understanding structures, let us consider a very simple molecule, Methane. The formula for methane is CH4. However, just ‘CH4‘ does not fully tell us about the molecular structure. The following formula gives us a better understanding of the structure –

So now we know that the central atom in methane is carbon and that it is attached to four hydrogen atoms. However, the above structure does not provide all the information about a methane molecule.
Methane is NOT a planar molecule, and all the atoms are not in one single plane. So, we need another structure that tells us how these atoms are arranged in space. The following structure helps us with that information –

In the above structure, we see that the carbon atom and two hydrogen atoms are in one plane(bonds shown with simple lines). One hydrogen atom is above the plane(bond shown with a wedge line), and one is below the plane (bond shown with a dotted line). This structure correctly represents the methane molecule and shows us the tetrahedral structure of this molecule.
This was a very simple example, but as the structures start getting complicated, it becomes very important to correctly represent them. A little change in the spatial arrangement of an atom could change the properties of the molecule totally!
Let us begin studying how molecules are represented in chemistry.
The Condensed Formula
This form of notation uses text form only to represent molecules.
e.g. – Methane is represented as CH4.
Propane as CH3CH2CH3.
Rules for writing the condensed formula are as follows-
- Parentheses/Brackets are used to indicate multiple identical groups. These brackets simplify a long formula.
- e.g.– Decane whose formula is CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3, is written as CH3(CH2)8CH3.
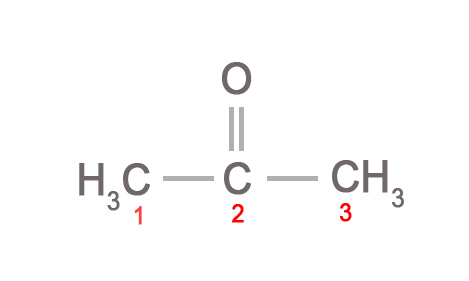
- Carbonyl oxygen atoms (C=O) can also be written in brackets.
e.g. CH3C(O)CH3 implies that the oxygen atom is attached to the second carbon atom.
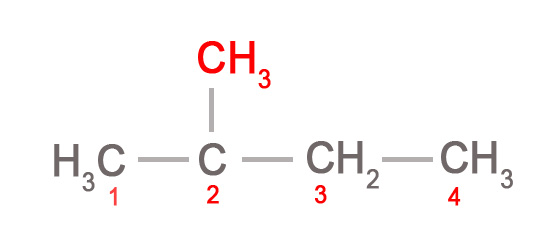
- Branching in hydrocarbon chains can also be depicted using brackets. The substituent on any middle carbon atom is written in brackets after that carbon atom.
- e.g.– CH3CH(CH3)CH2CH3. This formula indicates that the CH3 group in the bracket is on the second carbon atom i.e there is branching at the C-2 atom, as shown below.
Rule: Always look to the left of the bracket to see which atom it’s attached to.
Some more examples –
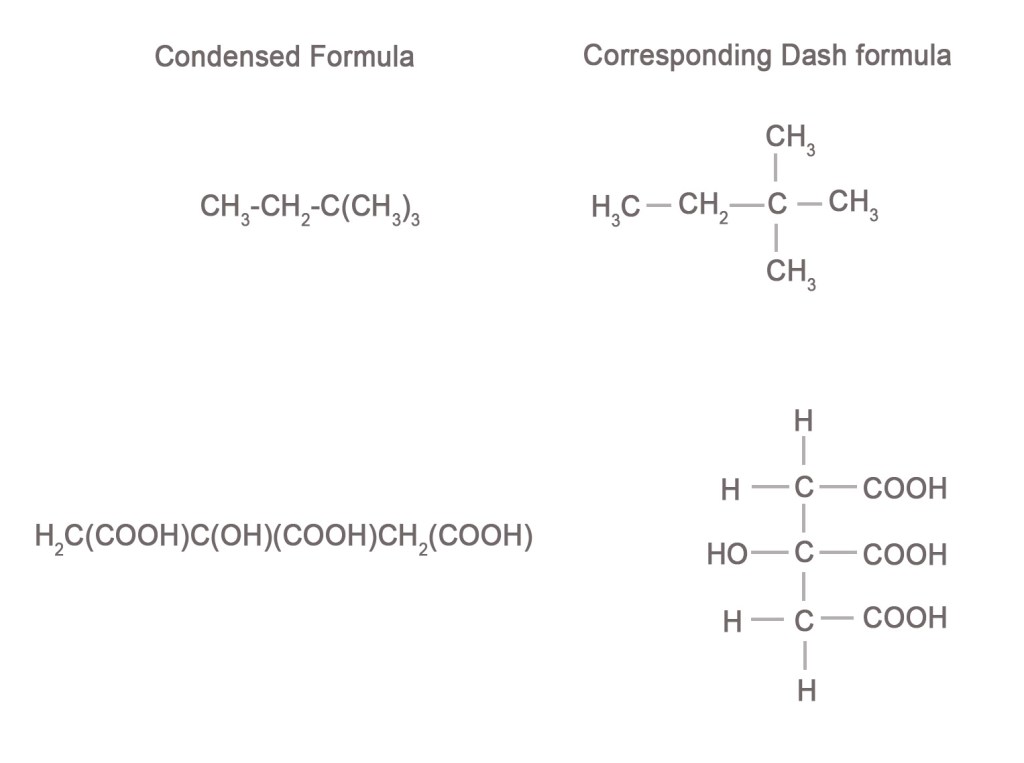
The condensed formula can be written for aliphatic compounds. However, it is not suitable for very complicated or cyclic structures.
Lewis Structures

Gilbert Newton Lewis was a member of the Royal Society and the chemist who introduced the concept of covalent bonds and Lewis/dot structures to represent covalent bonds. Lewis structures are a simple way of representing the valence electrons in a molecule.
Generally, the valence electrons are responsible for any chemical change that happens in a species. Thus, only the valence electrons are considered while drawing these structures. Valence electrons are represented by dots(•). Thus these are also called dot structures. The advantage of drawing these structures is that they tell us, the number of shared pairs and lone pairs of electrons, that exist in the molecule. Knowledge of this arrangement helps us understand the geometry of the molecules. We shall study more about this when we discuss the VSEPR theory.
Steps for formulating Lewis structures –
- STEP 1- Determining the total number of valence electrons in the molecule.
We begin by counting the total number of valence electrons in a molecule. We use the periodic table and electronic configurations of atoms to determine the valence electrons in each atom. Adding all values of valence electrons on each atom gives us the total number of valence electrons in that molecule.
no of valence electrons in a compound = ∑ (no of valence electrons on individual atoms).

# of valence electrons= Group number in the periodic table.
( Carbon is in group 4 so it has 4 valence electrons).
There are exceptions to the above rule. For example, helium(He) is in the 8th group of the periodic table but has only 2 valence electrons. The transition elements show multiple valencies too. For example, copper can exist as a cuprous ion(Cu+) or a cupric ion (Cu2+).
To understand this concept better watch – https://youtu.be/x1gdfkvkPTk
Now let us learn to find the number of valence electrons by taking an example.
e.g.- Acetaldehyde (CH3CHO).
# valence electrons in CH3CHO = 2(# valence electrons in carbon)+ 4(# valence electrons in Hydrogen)+ #(valence electrons in oxygen).
= 2(4) +4(1) + 6
= 18 electrons.
- STEP 2- Drawing the skeletal structure of the molecule.
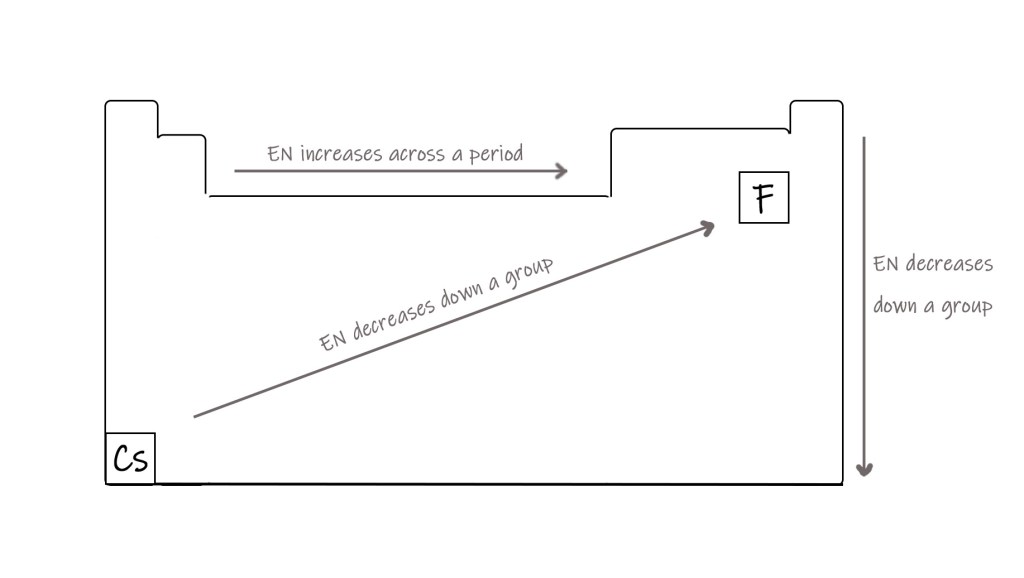
The next step in drawing lewis structures is to figure out the basic skeleton of the molecule. This is done by placing the least electronegative atom in the center (except hydrogen for organic compounds). The central atom should be the one that can form the highest number of bonds, or can have an expanded octet.
We have studied the periodic trends in electronegativity in post 46. The electronegativity shows the following trend-
F > O > Cl > N > Br > I > S > C > H > metals
So, for a molecule like NO2, nitrogen will be in the center, flanked by two oxygen atoms –
O-N-O . This is because nitrogen is less electronegative than oxygen, and is trivalent. Oxygen, on the other hand, is divalent. Thus, we arrange the atoms properly to get a rough structure of the molecule.
- STEP 3- Assign electrons to each atom.
Once a rough skeleton is drawn, we draw the electron pairs on the more electronegative atom/s until their octet is complete. Then we do the procedure for the next electronegative atom/s.We keep on repeating this process till all valence electrons are drawn around the atoms in the molecule.

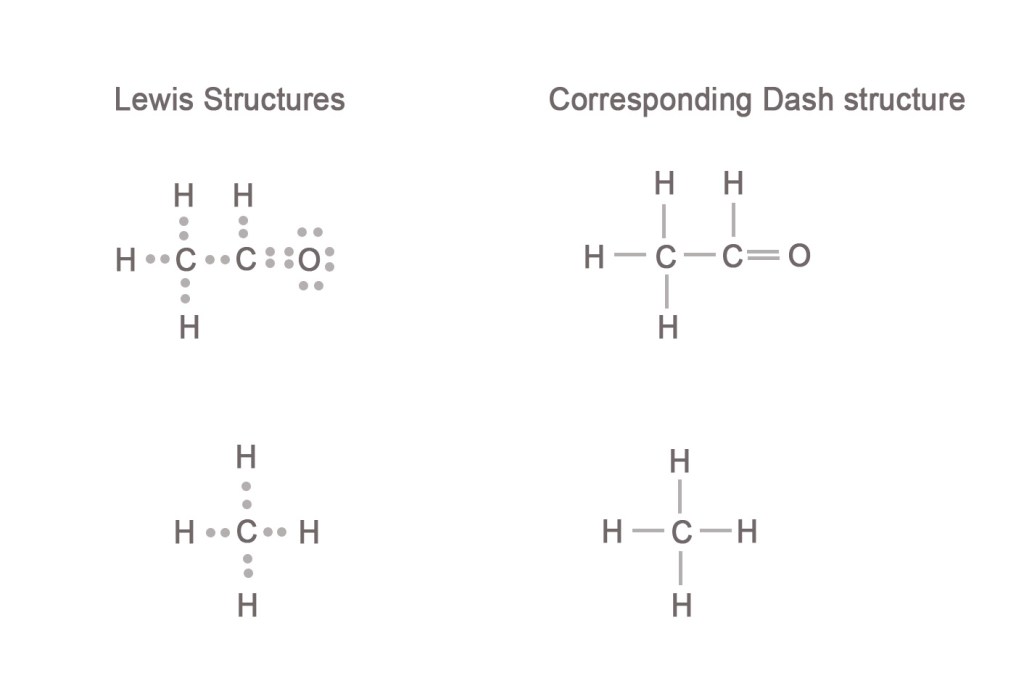
As seen in the above example, oxygen is the most electronegative of all elements present in the acetaldehyde molecule. Thus, we first complete the oxygen atom’s octet by correctly drawing the bonding pair and lone pairs of electrons on the atom. Then we start completing octets of the two carbon atoms. Thus, we arrive at acetaldehyde’s Lewis structure. The final structure has 18 electrons with octets of carbon and oxygen, and the duplets of all hydrogen atoms complete. The electron pairs on oxygen, which are not shared with any other atom, are the LONE PAIRS of electrons.
4 electrons are shared between carbon and oxygen indicating that there is a DOUBLE BOND between them.
- STEP 4- Find formal charges on atoms, if any.
Not all atoms in a molecule are neutral. Some atoms may have a formal charge (positive or negative). Calculating the formal charge on the atoms is the next necessary step. To know more about Formal charges read post 104. The most suitable lewis structure is the one with octets of atoms complete and the least formal charge on all atoms.
The Dash Structures
Two electrons shared between adjacent atoms represent one bond in the dash structure formula, as shown below. A double bond has four and a triple bond has six shared electrons.
Here are some more examples –
The Skeletal formula
These formulae are like shortcut structures to the dash structures of organic molecules. They show us the skeleton of the molecule. So, these kinds of formulas are useful in writing complex molecules, as they simplify those structures for us. It is very important to practice writing these, as they can get a bit confusing.
The concept of skeletal structures was introduced by German Chemist, Kekulé – the man credited to have found the benzene structure! Read more about the Kekulé benzene structure here.
Skeleton structures use only shapes to represent molecules. It takes a little practice to intuitively understand these structures. Let us learn to draw these with some examples.
e.g.- Consider the propane( CH3CH2CH3) molecule.This is a hydrocarbon with three carbon atoms arranged in a row. The dash and skeletal structures of this molecule can be drawn as follows-

Both the above structures represent the same molecule.
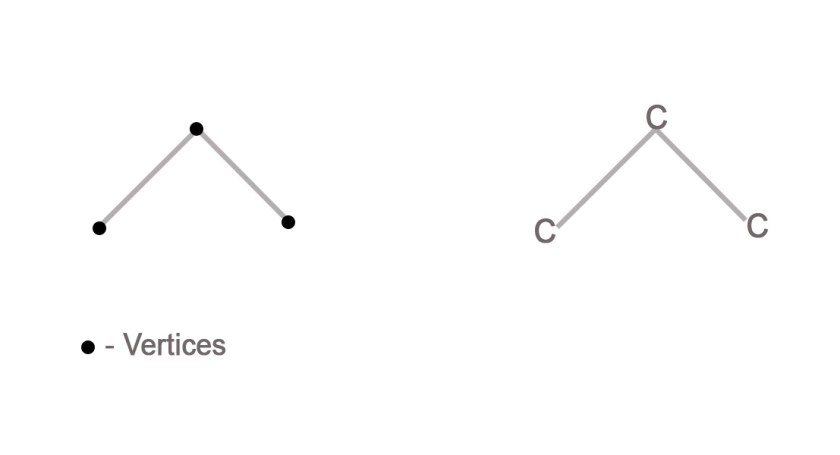
To make sense of the skeletal structure, let us count the number of vertices in it. The skeletal structure has three vertices (shown by dots in the adjacent figure ). These vertices are the three carbon atoms in propane.

As chemists, we are expected to know that carbon is tetravalent. So, the two terminal carbon atoms shall have three hydrogen atoms on them, and the middle carbon atom shall have only two.
The general rules for drawing skeletal structures are –
- The Carbon atoms– The carbon atoms are assumed to be at the vertices of the line segments of aliphatic compounds. For aromatic/closed chain compounds, the carbon atoms are assumed to be at the vertices of the respective shape. e.g. A benzene molecule is represented as a hexagon with alternate double or single bonds/a circle in the center.
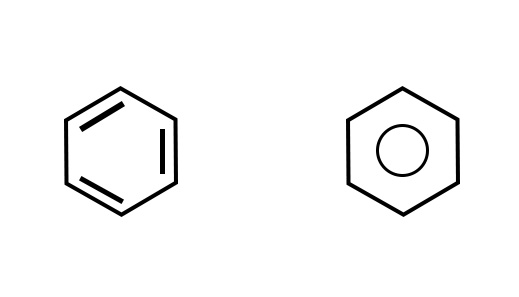
It is understood that carbon atoms are positioned at the vertices of the hexagon and two hydrogen atoms are attached to each carbon as shown below-

- The hydrogen atoms – Whenever there is no substituent on the carbon atom, it is supposed that the tetravalency of the carbon atom is fulfilled by hydrogen/s.
- Substituents on the carbon atoms – Any substituents on the carbon atoms are also shown with skeletal structures.
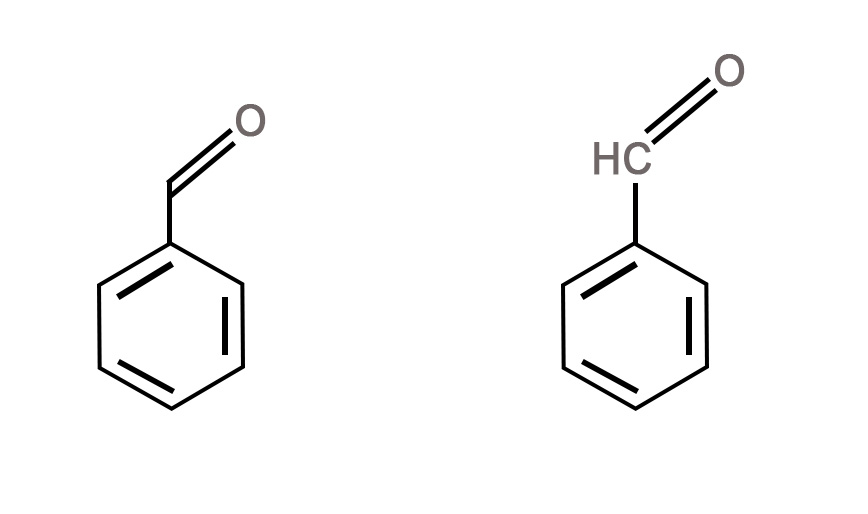
- The lone pairs – The lone pairs are not shown in the skeletal structures. We are expected to know where they are present in the molecule.
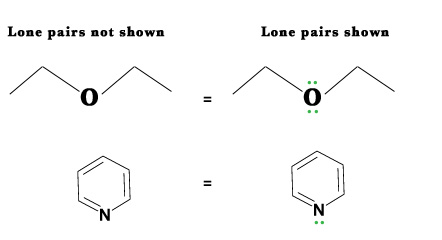
- The formal charges – The formal charge on a species is not shown if there is an ironic end to an organic molecule.

Some more examples –

There is a lot more to structures in organic chemistry. Depicting the correct spatial arrangements of atoms is a whole new chapter called stereochemistry. We shall study the organic structures in much greater detail then.
In this post, we have only discussed the basic structural representation of molecules. We shall keep learning more. Till then,
Be a perpetual student of life and keep learning…
Good day!
References and further reading –
1.http://www.masterorganicchemistry.com/2011/06/20/deciphering-what-the-brackets-mean/
2.https://en.wikipedia.org/wiki/Structural_formula
Image source –
1.https://www.alphachisigma.org/page.aspx?pid=486
2.By Unknown – http://ihm.nlm.nih.gov/images/B16099 Uploaded by en:User:Maximus Rex., Public Domain, https://commons.wikimedia.org/w/index.php?curid=167877