This topic is sort of a link between the earlier topic we discussed(Resonance) and the upcoming topic we shall start discussing soon. The structure of the benzene molecule provides a great insight into many fundamental concepts in organic chemistry. The benzene ring is the parent structure in many molecules and thus a detailed understanding of its structure will help us comprehend many topics in organic chemistry.
Benzene was discovered by Michael Faraday in 1825. He isolated a new hydrocarbon from an illuminating gas, which he called “bi carburet of hydrogen“. Later, in 1834, a German chemist Eilhardt Mitscherlich (University of Berlin) synthesized the same compound by heating benzoic acid with calcium oxide-

Using vapor density measurements, Mitscherlich further showed that benzene has the molecular formula C6H6. This formula was surprising as it has an equal number of carbon atoms and hydrogen atoms. Not many known compounds had this 1:1 C: H ratio. This ratio also indicated that benzene was a highly unsaturated molecule, however, it showed exceptional stability! Thus, the chemists realized that they were looking at a unique molecule. Many different structures of benzene were predicted but none of these could properly explain the properties of this molecule.
August Kekulé and his dream!

August Kekule was a German scientist who had already shown that carbon is tetravalent and it possesses the property of catenation i.e carbon atoms can link to each other and form chains. However, he could not explain the benzene structure. One day, he dreamt of carbon atoms dancing around as strings, moving in a snake-like fashion. Then he saw the snake eating its own tail! Thus, it suddenly dawned upon him, that the structure of benzene was cyclic!
At an event in Berlin, in 1890, he recalled how he came up with the structure of benzene-
There I sat and wrote for my textbook; but things did not go well; my mind was occupied with other matters. I turned the chair towards the fireplace and began to doze. Once again the atoms danced before my eyes. This time smaller groups modestly remained in the background. My mental eye, sharpened by repeated apparitions of similar kind, now distinguished larger units of various shapes. Long rows, frequently joined more densely; everything in motion, twisting and turning like snakes. And behold, what was that? One of the snakes caught hold of its own tail and mockingly whirled round before my eyes. I awoke, as if by lightning; this time, too, I spent the rest of the night working out the consequences of this hypothesis.
The Kekulé quote is taken from the biographical article of K.
Hafner published in Angew. Chem. Internat. ed. Engl. 18,
641–651 (1979).
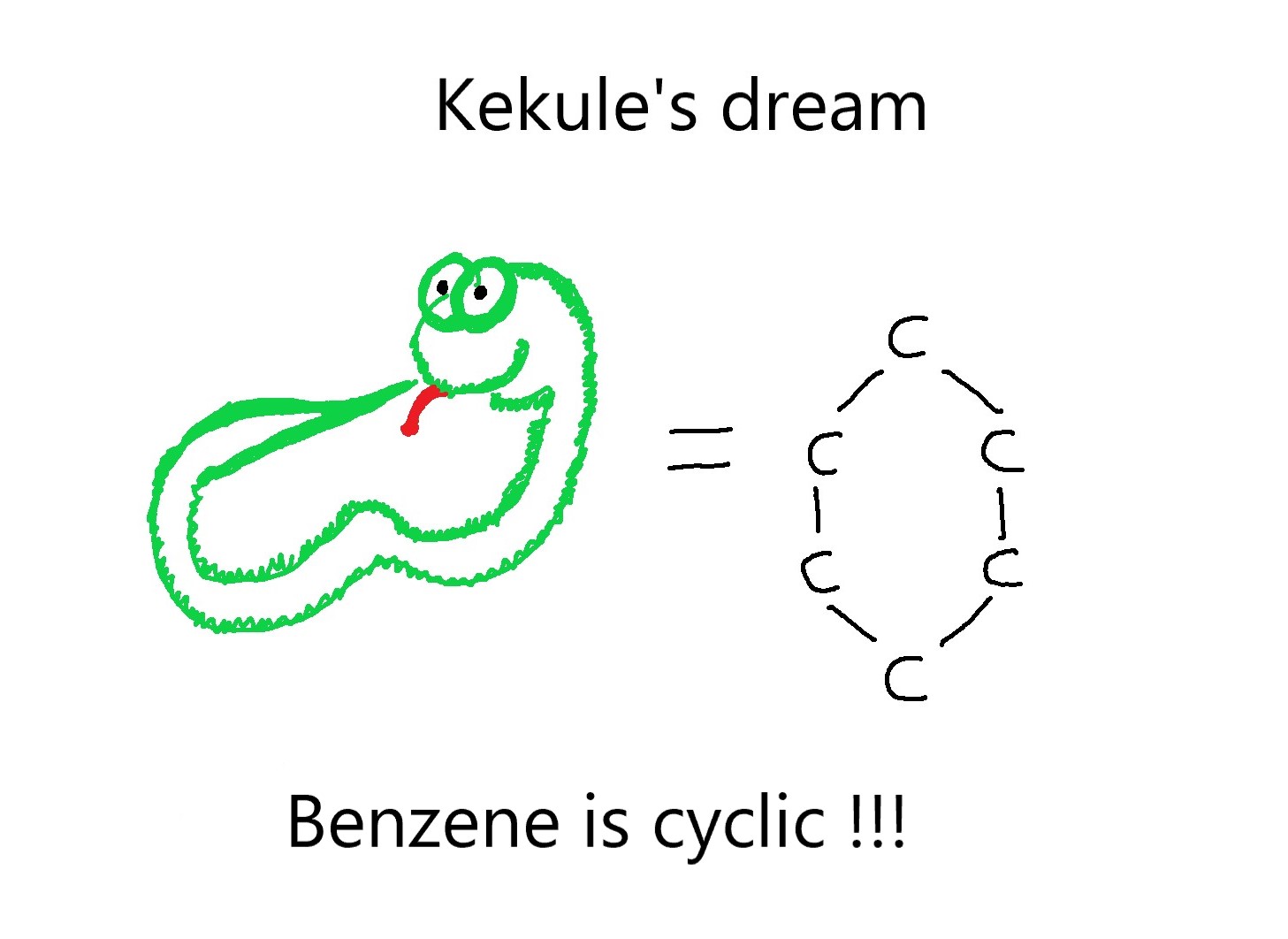
Thus, the structure of benzene was discovered!
All the carbon atoms in benzene are sp2 hybridized.

In the ground state, Carbon (6) 1s2 2s2 2p2.
In the excited state, Carbon (6) 1s2 2s1 2p3.

Each carbon is attached to another carbon atom and two hydrogen atoms. There are both σ and π bonds in benzene –
σ bonds in benzene –
As seen above, each carbon atom has three sp2 hybridized orbitals which form 3 σ bonds –
1)one sp2 – sp2 σ bond with an adjacent carbon atom and
2)two sp2 – s σ bonds with two hydrogen atoms.
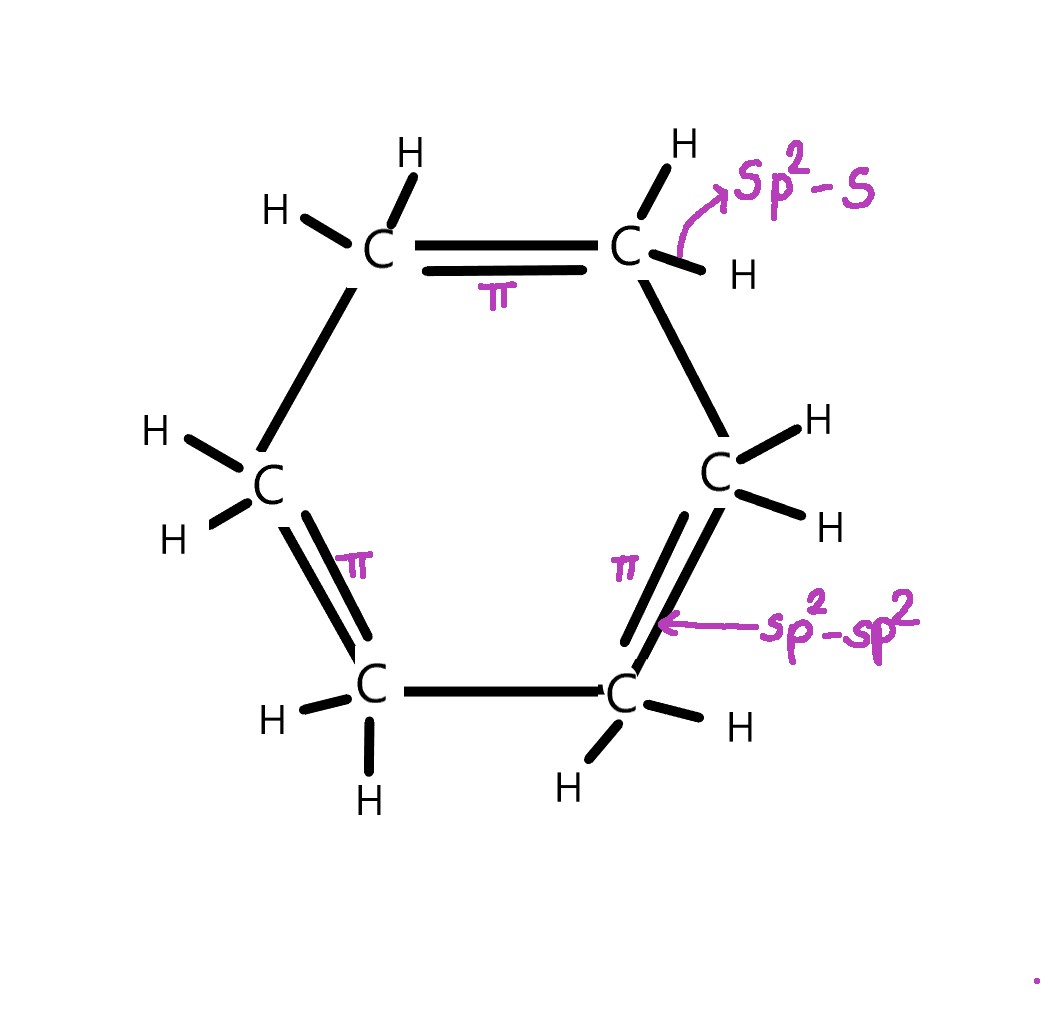
π bonds in benzene–
The unhybridized p- orbitals of each carbon atom overlap laterally, above and below the plane of the benzene ring. The π electrons are delocalized i.e spread overall carbon atoms. Thus, a π electron cloud appears above and below the ring.

It is important that we understand how the π electrons are observed both above and below the ring. The p-orbitals (unhybridized), that are responsible for the formation of π bonds are dumbbell-shaped. They lie perpendicular to the σ framework. One lobe of the dumbbell lies above and the other lies below the benzene ring.

The electrons constantly are in motion in these orbitals and they can either be in the lobe above the ring or the lobe below the ring. As all electrons keep moving constantly, at a given instant collectively, we see an electron cloud above and below the ring.
Benzene is a flat molecule, with every carbon and hydrogen lying in the same plane. It is very symmetrical, with each carbon atom lying at the angle of a regular hexagon. Thus, every bond angle is 120º.
In the next post, we shall continue discussing this structure in detail.
Be a perpetual student of life and keep learning…
Good day!
Image source –
1. [http://en.wikipedia.org/wiki/File:Benzene_Orbitals.svg Wikimedia Commons]. Reused under the [http://creativecommons.org/licenses/by-sa/3.0/deed.en Creative Commons Attribution-ShareAlike 3.0 Unported (CC BY-SA 3.0) license]. Attributed to [http:/
References and further reading –
- Organic Chemistry by Carey- 4th edition.
One comment