In the last post, we studied what resonance means and how resonating structures blend to give the resonance hybrid structure. In this post, we shall further look into the concept of resonance.
Facts about resonating structures –
1)All the resonating structures are imaginary and have no real existence.
2)All resonating structures contribute to the actual structure. The contribution by each structure may not be equal.
e.g. HCl molecule –
As seen in the figure below, HCl has three resonating structures. The difference in these structures is where the electrons lie. The last structure is the poorest contributor as a negative charge resides on the hydrogen (the more electropositive element) and a negative charge resides on chlorine (the more electronegative element).

3)The number of unpaired electrons in all resonating structures should be the same.
As seen in the figure below, the last structure has two unpaired electrons. As no other structure has an unpaired electron, this last structure cannot be considered a contributor to the overall structure of the oxygen molecule.

4)Resonating structures should have almost the same energy.

5)Similar charges should not reside on neighboring atoms and unlike charges should not be widely separated.

6)Relative order of atoms in a molecule should be the same while drawing the resonating structures.

7)Greater the # of covalent bonds, the greater is the stability. Thus, the resonating structures involving π bonds always contribute more towards stability.
8)More the # of resonating structures greater is the stability of the molecule.
RESONANCE ENERGY – The difference in energy between the actual molecule and the most stable canonical/resonance structure is called resonance energy. More the resonance, more the resonance energy. We expect a certain value of the energy of a molecule when we do not consider the resonance factor. However, resonance in the structure results in a decrease in energy than expected. This decrease in energy is referred to as resonance energy.

How do we use the concept of resonance?
This concept of resonance is very widely used in all branches of chemistry. Let us study an example to figure out how the concept of resonance helps us to study the bromination reaction of alkenes.
When ethene is treated with Br2/CCl4 mixture, we get an addition reaction. The bromine adds across the double bond yielding a dibromo product as follows –


If the same reaction is carried out, with 1 mole of bromine, on 1,3- butadiene, we would expect a 1,2 – dibromo product. As there is only 1 mole of bromine, it will attack the double bond either on the left/right and yield 1,2-dibromo butadiene as follows –
However, we get 1,4 -dibromo products too!
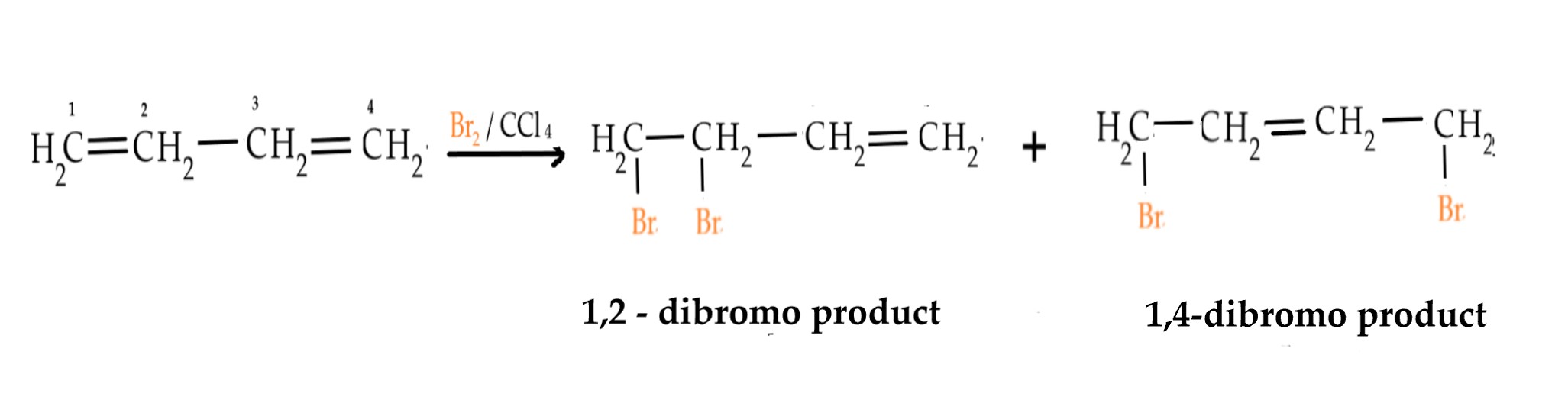
This can only be explained by resonance. The π electrons in 1,3-butadiene are delocalized over the entire molecule due to resonance and thus two products are obtained.
Now that we have an idea of what resonance is, we can proceed to discuss some important topics in organic chemistry.
Be a perpetual student of life and keep learning…
Good day!