In the last post, we introduced ourselves to a new concept in organic chemistry – Aromaticity. We will continue to discuss it in this post.
Hückel’s rule –

Erich Hückel was a German physical chemist, who was born in Berlin, Germany. His father was a physician, who had built a laboratory in their house. In this laboratory, he used to conduct experiments in chemistry and physics with his sons. This is how Erich Hückel developed his interest in chemistry. He is one of the most celebrated scientists of the 19th century. He is the one who gave us Hückel’s rule, Hückel’s molecular orbital theory, and Debye- Hückel theory.
Erich Hückel formulated a simple expression of the relationship between the structure of a compound and aromaticity as follows –
Hückel‘s rule
Planar monocyclic completely conjugated hydrocarbons will be aromatic when the ring contains 4n+ 2π electrons, where n ⇒ zero or any integer. Thus, rings with 2,6,10,14 etc electrons are aromatic.
Benzene has 6 π electrons and is thus aromatic.

Erich Hückel developed this rule to predict which systems can be termed aromatic. The rule applies to structures which –
i) have a cyclic (ring) structure.
ii) are planar i.e all atoms lie in the same plane. Thus, the atoms are sp2 hybridized. This ensures that there are continuously overlapping p- orbitals.
iii) have 4n+2 π electrons
iv)π electrons delocalized over continuously overlapping p- orbitals.
e.g.- Benzene molecule – It has a planar ring structure. All carbon atoms are sp2 hybridized. The unhybridized p- orbitals lie perpendicular to the σ framework as studied in post no 106 and the electrons in them are delocalized (i.e they are not localized on one single carbon atom). They form an electron cloud as shown in the figure below. The electrons can be in the upper lobe or lower lobe of the p-orbital. The electrons in the upper lobe and lower lobes form upper and lower π electron clouds respectively as shown in the figure below.
Benzene has 6 π electrons, which means it satisfies Huckel’s rule. Thus, all these features make it an aromatic structure.

ARE THE FOLLOWING COMPOUNDS AROMATIC OR NOT?
- Cyclopentene –
A: This is a planar ring, however, there are no conjugated double bonds.
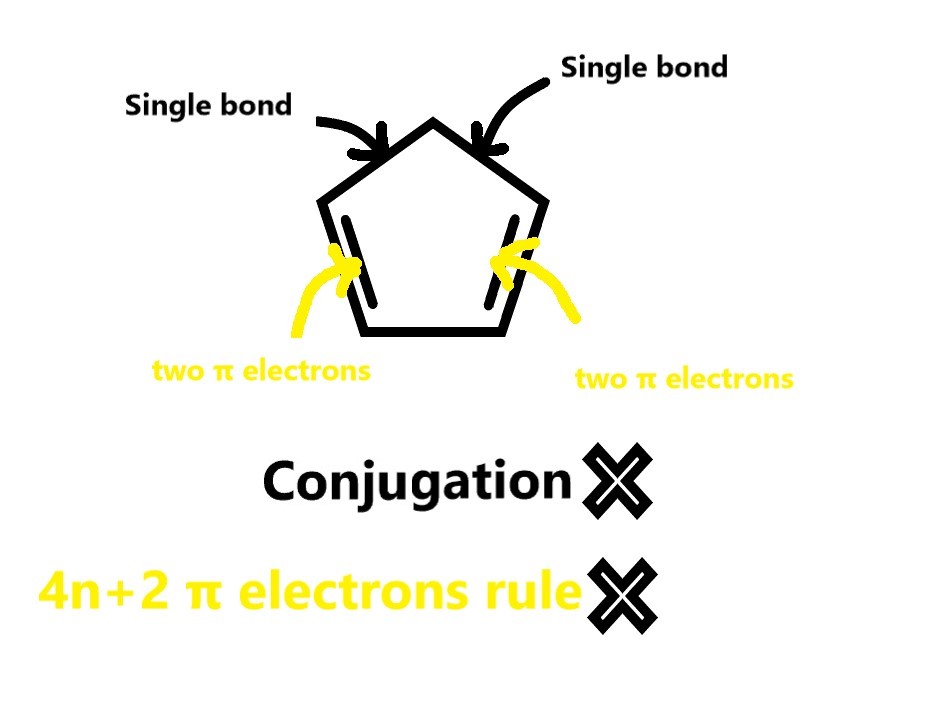
The no of π electrons =2×no of double bonds = 2× 2 =4. Thus, this does not follow the 4n+2 π electron rule. So, this structure is NOT aromatic.
2.

A: This structure looks unfamiliar and a bit complicated. However, we just have to see whether or not it is aromatic. Let us see if it obeys Huckel’s rule or not. As seen above all the atoms are in the same plane. Thus, this is a planar structure.
4n+2 = 4(2)+2= 8+2=10.
So, this molecule has 4n+2 π electrons and it also has conjugated double bonds. Thus, this molecule is aromatic.
3.

A: The above molecule is planar and also has conjugated double bonds. However, it does not have a cyclic structure and is NOT aromatic.
In the next post, we shall study the Huckel molecular orbital theory. Till then, be a perpetual student of life and keep learning….
Good day!
References and further reading –