Benzene with six π electrons – the aromatic sextet– is a “perfect” example of an aromatic compound. In this post we shall study some examples of homocyclic aromatic compounds, other than benzene.
Aromaticity of carbocyclic/homocyclic systems.
What is a carbocyclic system?
Homo = same (all carbon atoms)
Cyclic = ring structure.
A chemical species containing a closed ring of carbon atoms is called a homocyclic or carbocyclic system.
e.g.

All the above compounds have a ring structure and have ONLY carbon atoms in their ring. Thus, they are carbocyclic systems. However, not all of them are aromatic.
Let us study examples of carbocyclic systems, which are aromatic – Cyclopentadienyl anion (C5H5–) and cycloheptatrienyl/tropylium cation (C7H7+).
1.CYCLOPENTADIENYL ANION(C5H5_).
When a proton is removed from cyclopentadiene, the resulting anion has a pair of electrons on carbon atom 1.

Hybridization of C1 changes from sp3 to sp2 on loss of a proton. (Note that all other carbon atoms except C1 are sp2 hybridized). Now the pair electrons occupy the vacant p- orbital on C1 –

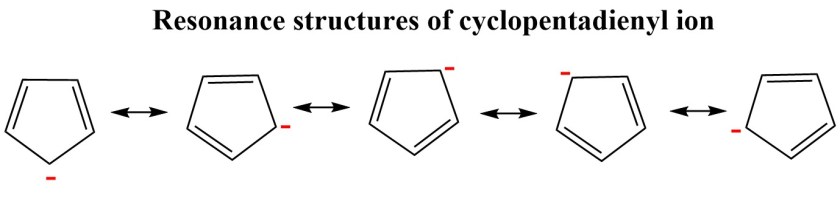
The above anion has 6 π electrons in five p- orbitals. Thus, this is a 6π electron system just as benzene. The atomic p- orbital on carbon atom 1 can overlap with four atomic p- orbitals of the original diene system. So, the six π electrons are delocalised over the entire cyclopentadiene ring and the anion can be represented as follows –

This anion is AROMATIC as it has (4n+2) π electrons. All the carbon atoms are sp2 hybridized and thus this ring structure is planar. The ball and stick model of this species can be shown as follows –

Acidity of cyclopentadienyl ion-
Removal of a proton from cyclopentadiene results in cyclopentadienyl anion.

Acids have an ability to donate a proton easily( we will study acids and bases in detail in the upcoming posts ). Just because this ion is so stable, cyclopentadiene is much more acidic(pka=14) than a simple diene. Five contributing resonance structures show that each carbon atom carries 1/5th of the negative charge.
Spectral data –
The IR and Raman spectra of this anion are simple and suggest a symmetrical planar structure. The electronic spectrum shows no absorption above 200nm and the NMR shows a singlet at δ=4.57ppm. The spectral data too suggests that the anion is planar and aromatic.(We will understand the spectral information better when we study spectroscopy in detail).
2. CYCLOHEPTATRIENYL/TROPYLIUM CATION (C7H7+) –
When treated with triphenyl carbonium fluoroborate (Ph3+CBF4–), cycloheptatriene forms a very stable cycloheptatrienyl / tropylium cation.

This species also has 6π electrons in conjugation and is AROMATIC.
The IR and Raman spectra of this cation are consistent with a symmetrical planar structure. The electronic spectrum shows a max at 275nm and NMR spectrum has a singlet at δ=9.28 ppm.
The ball and stick model can be represented as follows –

The tropylium cation is a regular heptagon( angles 128.5º).Six carbons contribute one p electron each and the seventh contributes only an empty p orbital.
As a general rule –
Polar molecules (which have polarity i.e + and – in them) dissolve in polar solvents.
Non-polar molecules dissolve in non-polar solvents.
Water is a polar solvent as it can dissociate as H+ and OH–.So,only polar molecules dissolve in water.
Alkyl halides such as cyclopentyl chloride are nonpolar and dissolve in non-polar solvents. Thus, they remain insoluble in water.
However, cycloheptatrienyl bromide shows an exception. It dissolves in water. This is because, cycloheptatrienyl bromide is an ionic compound.
Ionic compounds dissociate in water to their respective anions and cations. As the tropylium cation is aromatic , it shows exceptional stability in water. In cyclohepatriene , all carbon atoms are sp2 hybridized except one. One carbon atom is sp3 hybridized and thus does not have a vacant p-orbital. Being sp3 hybridized , it has a tetrahedral geometry( i.e it is NOT planar). Thus, this molecule is NOT aromatic.

Like cyclopentadienyl anion , tropylium cation does not undergo electrophilic substitution reactions, yet both ions must be considered aromatic according to all other criteria for aromaticity (given by Hückel‘s rule).
Other charged aromatic species are –

3. CYCLOPROPENIUM CATION –

This cation shows exceptional stability. It has 2 π electrons. Thus, it has 4n+2 π electrons(n=0). It is planar and the 2 π electrons can move in the vacant p-orbital of the carbon atom having positive charge. The positive charge indicates that the electron from that orbital has been removed and thus it is vacant.

The 1,2,3-tri-t-butylcyclopropenium cation is so stable that the perchlorate salt can be recrystallized from water.119 An X-ray study shows that the carbocation exist as a discrete ion in the solution.
4. CYCLOBUTADIENE DICATION –
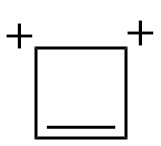
This dication has 2 π electrons. Thus, it has 4n+2 π electrons(n=0). It is planar and 2 π electrons can move in the two vacant p-orbitals of carbon atoms with a positive charge. Thus, this dication is considerably stable as opposed to other dications which are very unstable and difficult to make.

In the next post we will study some more aromatic compounds. Till then,
Be a perpetual student of life and keep learning…
Image Source –
1.By Benjah-bmm27 – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=2063771
2.By Ben Mills – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=3400770