In the last post we studied naphthalene molecule in detail and we understood why it shows aromatic stability. Similarly , other polycyclic hydrocarbons are also aromatic in nature.
Azulene.

The name azulene comes from the Spanish word ‘Azul‘ which means blue.
Azulene is a bicyclic aromatic hydrocarbon containing a five – and seven- membered ring fused together. It is an isomer of naphthalene i.e it has same number of carbon and hydrogen atoms but their arrangement is different. Naphthalene is white colored whereas azulene is a dark blue colored solid. This is a classic example of how the properties change drastically when the structure changes!

It is a non-benzenoid aromatic compound – a compound which does NOT have a benzene ring in it.This compound is derived from steam distillation of chamomile plant. Azulene is known for its soothing and anti-inflammatory properties and is widely used in shaving creams, bath salts and creams.
Resonance energy of azulene is 205 kJ /mol. The resonance structures can be shown as follows –

The second resonating structure can be thought of as fusion of cycloheptatriene and cyclopentadiene rings.There is a negative charge on the cyclopentadiene ring and a positive charge on cycloheptatriene ring. This dipolar nature gives azulene its blue color.
Anthracene and Phenanthrene
Like naphthalene and azulene, Anthracene and phenanthrene are isomers too. In anthracene, three rings are fused together linearly , while in phenanthrene they are fused angularly .Both are benzenoid structures.
 The resonance structures for anthracene can be drawn as follows –
The resonance structures for anthracene can be drawn as follows –

The resonance energy of anthracene is 84 kcal/mol and that for phenanthrene is 92kcal/mol. For benzene the resonance energy is 36kcal/mol. However,
Stability of the PAH α resonance energy per benzene ring.
Thus, resonance energy per ring for anthracene(3 rings) = 84 ÷ 3 = 28kcal/mol.
and resonance energy per ring for phenanthrene (3 rings) = 92 ÷ 3 = 30.67 kcal/mol.
As both these energies are less than the resonance energy of benzene, benzene is more stable than anthracene and phenanthrene.
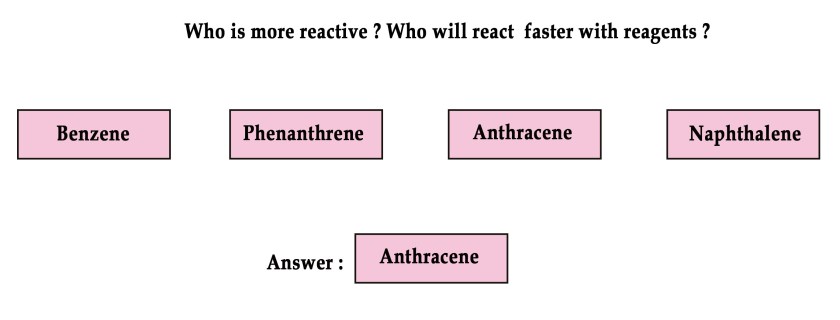
More stable means less reactive .
Thus,
Order of stability ⇒ Phenanthrene > Phenanthrene > Naphthalene > Anthracene.
Order of reactivity ⇒ Anthracene > Naphthalene > Phenanthrene > Phenanthrene.
Pyrene.
Pyrene is an aromatic compound too. It has 4 rings fused together. This molecule is planar. It has 8 double bonds. The 16π electrons are delocalised over the entire structure and this structure shows extra stability.

An interesting research on this molecule is of special interest. In this research, scientists studied a particular Kekulé structure of pyrene with a double bond in the centre. Pyrene has 16 π electrons , which is a non- Hückel number. However, they learned that , if the central double bond is ignored and only the atoms on the periphery are considered , then the resulting structure would be planar , monocyclic with 14 π electrons – which is a Hückel number !! (4n+2 , if n=3 , then 4(3)+2 = 14), in short the resulting structure would be aromatic.
 The resulting structure would be an annulene structure as follows –
The resulting structure would be an annulene structure as follows –

Phenacenes
Phenacenes are a family of ‘graphite ribbons‘ in which benzene rings are fused together in alternating pattern. Phenanthrene is a simple member of this family.

The inner rings of phenacenes are more aromatic than the outer rings. They are actually more aromatic than benzene too !!
In the next post we will learn more about these class of compounds called ‘annulenes’. Till then,
Be a perpetual student of life and keep learning….
Good day !
References and Further reading –
1.March’s Advanced Organic Chemistry by Jerry March 6th edition.
Image source –



Its a very nice and informative post…very heartening to see you always keeping in touch with chemistry.. good luck with your endavour
LikeLike
Thank you so much Aditya 🙂 I am glad you are reading my posts 🙂
LikeLike