In this post we shall discuss aromaticity of some polycyclic aromatic hydrocarbons (PAHs).
What are polycyclic aromatic hydrocarbons?
Poly = many
cyclic = ring structure.
Polycyclic aromatic compounds –
- have multiple aromatic rings in their structure.
- have only carbon, hydrogen atoms in their structure
- the π electrons are delocalized.
e.g.-

These compounds show many properties linked with aromaticity.
Naphthalene.

The moth balls used commonly are actually naphthalene balls. We all know they have a characteristic smell. Although it is advisable NOT to use these, as they are carcinogenic.
Does naphthalene satisfy the conditions to be aromatic?
1)Naphthalene is planar.Naphthalene has two rings fused together with 10 carbon atoms. All the carbon atoms are sp2 hybridized. This molecule has 10 p-orbitals over which can overlap. Thus , the electrons can be delocalized over both the rings.
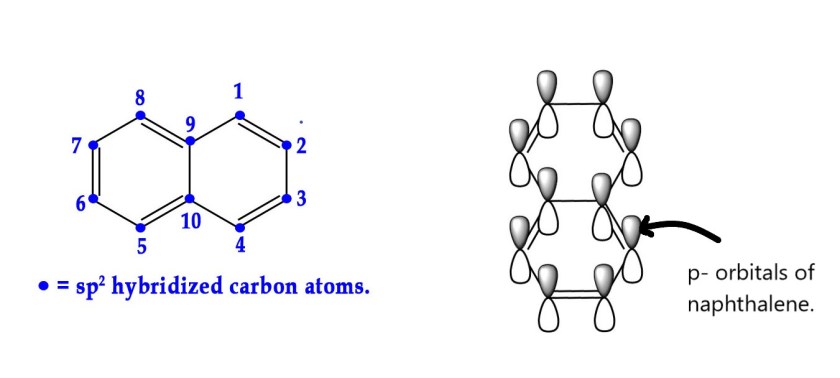
C-9 and C-10 in the above structures are called points of ring fusion. These catbon atoms bear no hydrogen atoms.
2)According to resonance theory, naphthalene can be considered a hybrid of three Kekulé structures –

As seen above, the π electrons are delocalised over both the rings. However, not all double bonds are in conjugation. Only one of the two rings has conjugation (alternate single and double bonds).
3)
Huckel’s rule applies only to monocyclic compounds. We cannot use it for polycyclic hydrocarbons.
Naphthalene has five double bonds i.e 10 π electrons. If we substitute n=2 it satisfies the Huckel’s rule – 4n+2π electron condition. However, technically this cannot be considered as a proof as this rule applies ONLY TO MONOCYCLIC compounds.
4)The heat of hydrogenation calculation also show stabilisation in the molecule. We have already seen in post 117 that heat of hydrogenation of cyclohexene to cyclohexane is -26.8 kcal/mol.
If heat released by hydrogenation of 1 double bond = – 28.6 kcal/mol , then,
heat released by hydrogenation of 5 double bonds(naphthalene) should be -28.6 × 5
= -143 kcal/mol.
However, the heat of hydrogenation of naphthalene calculated experimentally is 80 kcal/mol.
∴ Stabilization energy = -143-(-80) = -63kcal/mol.

5)Naphthalene shows substitution reactions with electrophiles rather than addition reactions , just as benzene.

All the above points clearly indicate that naphthalene is an aromatic entity too. It is not as aromatic as benzene, but it is aromatic nonetheless.
Resonance/stabilization energy of benzene = 36kcal/mol.
Resonance/stabilization energy of Naphthalene = 63 kcal/mol.
Then why is benzene more stable/ aromatic than naphthalene?
As seen above , the resonance energy of naphthalene(63kcal/mol) is more than that of benzene(36kcal/mol).So, naphthalene should be more stable than benzene. However, we see exactly the reverse trend here! Benzene is more stable than naphthalene. How do we explain this?
For PAHs ,
Stability of the PAH α resonance energy per benzene ring.
So, for naphthalene , the resonance energy per ring = 63 ÷2 = 31.5 kcal/mol, which is less than that pf benzene.
Thus, benzene is more stable than naphthalene. Technically , naphthalene has fused rings unlike benzene and thus the two systems are different and cannot be compared.
In the next post we will discuss some more PAHs. Till then,
Be a perpetual student of life and keep learning…
Good Day !
Image source –
1.https://www.top10homeremedies.com/news-facts/10-hidden-dangers-in-your-home.html
