Let us now begin to discuss the magnetic properties of aromatic compounds.
3]Magnetic properties.
I) Aromatic ring current–
Aromatic compounds have delocalised , closed loop of π electrons. These electrons form π electron cloud above and below the plane of the ring. We have already seen such cloud for benzene.
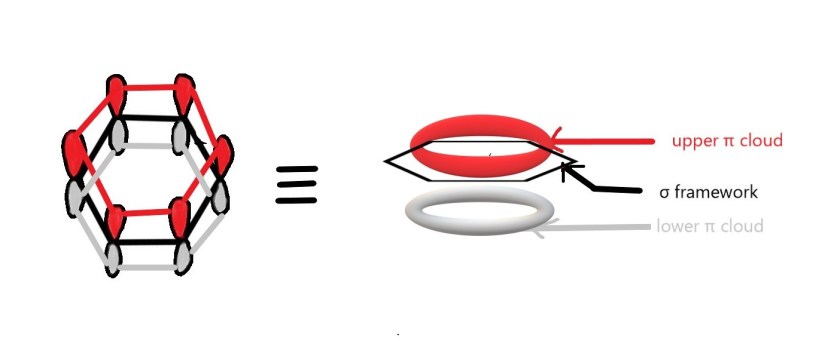
When such compounds are immersed in an external magnetic field, these π electrons begin to circulate(they are delocalised and thus can circulate).This circulation is called as ring current.
Paramagnetism ⇒ shown by species with unpaired electrons ⇒ attracted by magnetic field.
Diamagnetism ⇒ shown by species with no unpaired electrons ⇒ repulsed by external magnetic field.
We have seen in post 130 , that aromatic compounds do not have unpaired electrons. Thus, the ring current they produce is diamagnetic.
In contrast to this, anti-aromatic compounds have unpaired electrons( see post 130) .So, they exhibit a paramagnetic ring current.
So, aromatic compounds have a diamagnetic ring current and antiaromatic compounds a paramagnetic current and aromaticity can be defined as –
‘Ability to sustain an induced ring current’.
II)NMR technique –
The ring current is produced due to circulation of the π electrons. Electrons are negatively charged particles. When charged particles move , they produce their own magnetic field ( We all have learnt this in physics). Thus, the moving electrons too , induce a local magnetic field(Bind).When such a substance is placed in external magnetic field(Bo) as in a NMR spectrometer, the induced field interacts with the external magnetic field.(We will study NMR spectroscopy in detail later. For now just note that the aromatic compound- benzene – is subjected to an external magnetic field inside an NMR spectrometer).

As seen in the diagram above, there is a benzene molecule kept in an external magnetic field,Bo.
So, we have two magnetic fields –
| 1. |
External magnetic field, Bo |
applied to benzene from outside. |
| 2. |
Induced magnetic field, Bind |
produced in the benzene ring on account of the circulation of its π electrons i.e due to ring current. |
As shown in the figure, there are two zones/regions created on account of the interaction between two magnetic fields –
A)Shielded region – The region inside the cone ,where the Bo and Bind are in opposite directions i.e they oppose each other (see the red and green arrows)
and
B)Deshielded region – Where the Bo and Bind are in same direction.
Substituents on the aromatic ring that fall in the shielded region ⇒ will experience less magnetic field as the two fields are opposing each other in this region.
Substituents(-H in this case) in the deshielded region ⇒ will experience more magnetic field as both magnetic fields are acting on them in the same direction(additive effect).
In H-NMR spectra, the H’s attached to the ring appear downfield compared to their olefinic counterparts on account of deshielding effect. The NMR spectra is recorded as a scale of chemical shift(δ) from 0 to 14ppm.
Thus, aromaticity can be determined from NMR spectrum. The occurance of downfield shift of aromatic protons is an evidence for aromaticity.
III) Magnetic susceptibility –
Magnetic susceptibility is the measure of how much a material will become magnetized in an applied magnetic field. It can be determined by computational or spectroscopic methods.
It is observed that aromatic compounds(having delocalised electrons) have enhanced magnetic susceptibility (than localized structural components).This is called exaltation.
4]Hardness / HOMO-LUMO gap.
We will learn the chemical hardness and softness concept in detail when we study acids and bases. For the purpose of relating it to aromatic compounds, we just need to know that –
Hardness = (∈HOMO−∈LUMO)/2
HOMO ⇒ Highest Occupied Molecular Orbital.
LUMO ⇒Lowest Unoccupied Molecular Orbital.
From the above formula it is evident that, larger the HOMO- LUMO gap , greater is the hardness. Aromatic compounds have a large HOMO – LUMO gap.Thus, if the value of hardness is large it means that the compound could be aromatic.

What happens when the HOMO- LUMO gap is large?
As seen in the above figure, due to a large energy gap between HOMO and LUMO, the electrons in HOMO cannot be easily excited to LUMO. It will take a lot of energy to overcome this gap. So the HOMO electrons are not very reactive and the aromatic compound exhibits more stability. This results in reduced reactivity of aromatic compounds towards electrophilic reagents.
In the next post we shall study one more concept related to aromaticity. Till then ,
Be a perpetual student of life and keep learning….
Good day !