Q: Classify the following compounds as aromatic, anti-aromatic or non-aromatic –
1)

|
Is it cyclic ? |
✓ |
|
Conjugated double bonds ? |
✓ |
|
Is it planar? |
✓ |
|
# of π electrons |
8 |
|
# of lone pair of electrons involved in conjugation |
2 |
|
Total π electrons |
10 |
| 4n+2 ? |
✓ |
Thus, this compound is aromatic.
2)

In the above molecule, one carbon atom is sp3 hybridized and there is no complete conjugation in the molecule. This makes the molecule non-aromatic.
This molecule has 4n electrons yet it is NOT anti-aromatic. Why?
For a molecule to be anti-aromatic , it has to satisfy all conditions of aromatic compounds except that it needs to have 4n π electrons.
In this molecule, the single and double bonds are not conjugated. The sp3 carbon atom does not have unhybridized p- orbitals. So, continuous overlap of p-orbitals is not possible. Thus, this molecule is non-aromatic.

3)

This molecule is a carbocation (a carbon atom with a positive charge).In this species, the carbon atom with the positive charge is attached to 3 groups – two adjacent carbon atoms and a hydrogen. Thus, it is sp2 hybridized.

Thus, this carbon atom has a vacant p-orbital. As all carbon atoms are sp2 hybridized, a continuous overlap of p- orbitals is possible and thus the π electrons can be delocalised. However, this molecule has 4n π electrons. So, it is anti-aromatic.
4)

The above molecule has three heteroatoms, namely – two nitrogen and one sulphur. When sulphur atom has no formal charge, it has two lone pair of electrons and nitrogen has one lone pair of electron. We have studied the exception rule in post 61. The rule states,
If an atom –
I) has one or more lone pairs and
II)is attached to an sp2atom, then that atom is also sp2 hybridized.
In the above molecule, both nitrogen atoms are attached to sp2 carbon atom and have one lone pair. Thus, they are sp2 hybridized too. This means that they have 3 sigma orbitals, which form two bonds with two adjacent carbon atoms. The third sp2 orbital holds the lone pair and thus, we can conclude that the lone pair is NOT in the p-orbital. So, this lone pair will NOT take part in conjugation. Thus, the lone pairs on both nitrogen atoms will not be counted as π electrons. Sulphur, again according to the exception rule, is sp2 hybridized. However, in this case, one lone pair is in the sp2 orbital and other occupies the p π orbital. So, these two electrons take part in conjugation. The other 4 electrons come from two double bonds.
Thus, total no. of π electrons = 2+2+2 = 6.
| Is it cyclic ? |
✓ |
| Conjugated double bonds ? |
✓ |
| Is it planar? |
✓ |
| # of π electrons |
4 |
| # of lone pair of electrons involved in conjugation |
2 |
| Total π electrons |
6 |
|
4n+2 ? |
✓ |
Thus, this molecule is aromatic.
4)
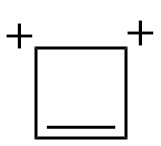 We saw this cyclobutadiene dication in post 121.
We saw this cyclobutadiene dication in post 121.
| Is it cyclic ? |
✓ |
| Conjugated double bonds ? |
✓ |
| Is it planar? |
✓ |
| # of π electrons |
2 |
| # of lone pair of electrons involved in conjugation |
0 |
| Total π electrons |
2 |
| 4n+2 ? |
✓ |
The molecule is aromatic.
In the next post we will solve some more problems based on aromaticity.Till then ,
Be a perpetual student of life and keep learning….
Good day !

exact explanation
LikeLike