Understanding the concept of photons is pertinent to the study of the second law of photochemistry. So, let us dive into this subject and learn all about photons!
The term photons comes from the Greek word phōtos meaning light.
Photons
All matter is made up of elementary/fundamental particles.
What are elementary/fundamental particles?
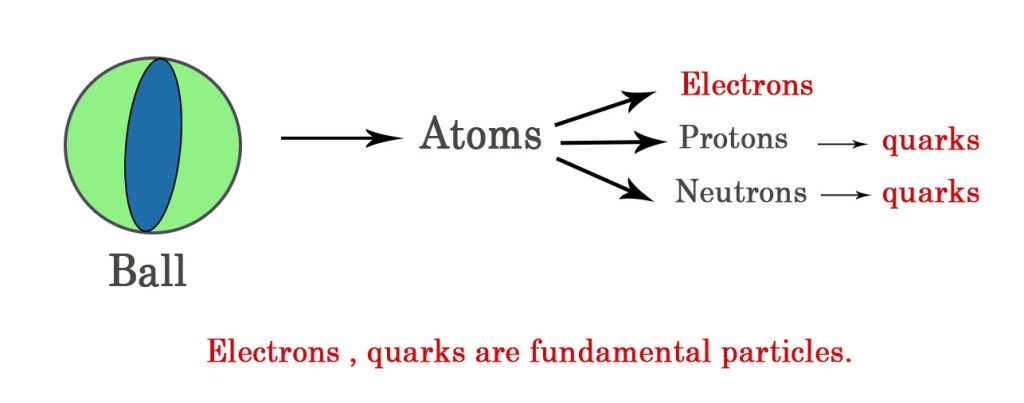
The smallest units of anything are called it’s elementary/fundamental particles. Fundamental particles DONOT have any internal structure and they are indivisible i.e they cannot further be divided into anything smaller.
e.g.– Consider a ball (matter) which is made up of atoms. However, an atom is divisible. We know, it consists of electrons, protons and neutrons. So, an atom is NOT a fundamental particle of matter. However, an electron cannot be further divided into any smaller unit. Thus, it can be termed as a fundamental particle.
The protons and neutrons in the nucleus are composed of quarks. This means that these sub-atomic particles can be further broken down into something more smaller- quarks.
However, quarks themselves are indivisible and thus they are fundamental particles. To know more about quarks and leptons read post 35.
In post 30 , we discussed the wave-particle duality theory, proposed by Louis Victor de broglie. According to that theory , waves can be modelled as particles i.e waves can be thought of as made up of many tiny particles.
The smallest elementary/fundamental particle of light is called a ‘photon’.


In our universe, we can broadly categorize everything as –
1)Matter and
2)Energy – more specifically light.
The difference between the two is REST MASS. Matter has rest mass whereas light doesn’t.

Now what is rest mass??
This concept was introduced by Einstein in his theory of relativity.
Rest mass(mrest) of an object is the mass of an object when it is at rest, from the point of view of the observer.
What does this mean?
An object will appear to be at rest to the observer, if –
i) the object is actually stationery or
ii)if the observer is moving at the same speed and direction as the object.
In both these cases, the mass of the object will be considered as it’s rest mass. In simple terms we can conclude, that rest mass is the mass of the object when it is at rest.
So, does this mean that the mass of an object changes when it moves? The answer to this question is YES !! As the velocity of an object increases , its mass also increases. However, for objects that travel with speed, significantly less than that of light ( which most objects do) , this increase in mass is very less and thus imperceptible. This mass of the moving object is called the relativistic mass (mrel).

The famous equation , E= mc2 , uses the relativistic mass. Thus, the equation can be precisely written as ,
Erel= mrelc2
Erel ⇒ Energy of the object
mrel ⇒ mass of the object
c⇒ velocity of light (3 × 108 m/s)
The velocity of light is a constant. So, we can conclude that, E is directly proportional to m. This means, higher the mass of the object , more energy is needed to move it. This seems very reasonable, isn’t it? It takes more energy to move a heavier object than a light one!
Coming back to the discussion on photons – we said that photons have no rest mass. This is because, photons are never at rest. Light, as we know, always propagates. So, photons are massless particles with discreet energy.
Photons are considered to be minute energy packets of electromagnetic radiation and this energy cannot be divided further. Moreover, this energy can be transmitted over vast distances without any decay in energy or speed!!Isn’t that cool? Imagine, the waves coming from the sun. The sun is so far away from the earth. Yet, the photons from the sun reach the earth without changing speed or energy they have.
A very important point to note here is that, the light energy is NOT continuously distributed over the wave but as discreet packets in form of photons.
Every photon of light has discreet energy which depends on its wavelength. The relationship between the energy of the photon(E) and the frequency (ν)/wavelength(λ) is given by the following equation –
E= hν = hc/λ
h is the planck’s constant.
When photons come in contact with matter, they can be absorbed by matter. They then transfer their energy to matter.
Now that we have a general idea of what photons are, we can start discussing the second law of photochemistry in the next post. Till then,
Be a perpetual student of life and keep learning….
Good day !