In the previous post we understood that hetero nuclear molecules are formed by two different atoms with similar energies. From this post onwards, we shall study some examples of heteronuclear molecules and learn how bonds are formed between two different atoms.
Hydrogen fluoride (HF)
Hydrogen Fluoride is the simplest known heteronuclear molecule. In this species, fluorine is more electronegative than hydrogen. The electronic configuration of hydrogen and fluorine atoms are-
H (1) – 1s1
F (9) – 1s2 2s2 2p5
For bond formation, the interacting orbitals must have comparable energy. However,the 2s orbitals of fluorine are way too low in energy (-40.2eV) than the 1s orbital of hydrogen (-13.6eV).
Why are the 2s orbitals of fluorine so low in energy?
The atomic number of fluorine is 9 i.e there are 9 protons in the nucleus of a fluorine atom. There is an electrostatic attraction between the protons in the nucleus and the electrons revolving around it. In a fluorine atom, the 1s and 2s electrons, which are near the nucleus, experience a very strong pull (more attraction) from the 9 protons in the nucleus. Therefore, they are tightly bound to the nucleus.
However, in an hydrogen atom, there is only one proton in the nucleus. So, the electrostatic pull experienced by the 1s electron of hydrogen is comparatively less. Thus, the electrons in the 1s and 2s orbitals of the fluorine atom are lower in energy than the 1s electron of H -atom.

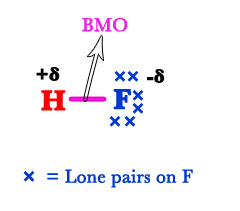
Only the valence electrons are responsible for chemical reactivity. Thus, while drawing MOT diagrams, we only consider the valance electrons. For the fluorine atom, the 2s and 2p are the valence orbitals. Therefore, only these two are shown in the figure below. The 2s electrons DO NOT take part in bonding. Thus, they are called nonbonding (nb) electrons. In classical terms, this is a lone pair of electrons. As this orbital does not interact with another orbital, its energy remains the same.

The 1s of the H-atom and 2p orbitals of the F-atom are comparable in energy. So, they interact with each other. However, only the pz orbital has the same symmetry (around the internuclear axis) as the s – orbital. As a result, the 1s of the H-atom overlaps with the 2pz orbital of the F-atom. The px and py orbitals remain unaffected as they lie in different planes. The electrons in these orbitals are nonbonding/lone pairs on the F-atom.

Thus, there are three lone pairs on the F-atom. The electrons in the bonding molecular orbital (BMO) shift more towards the F-atom. This is because the fluorine atom has more electron affinity than the hydrogen atom. This can be seen in the Lewis structure of the HF molecule. The δ+ charge indicates that the BMO electron pair is shifted away from H. The δ- charge indicates a partial negative charge developed on the F-atom due to the shifting of the electron pair shifts towards it.
We shall continue studying more MO diagrams in the upcoming posts.Till then,
Be a perpetual student of life and keep learning …
Good Day!
Thank You. You really helped me.
LikeLiked by 1 person
My pleasure 🙂
LikeLike